Serological Indicators and Survival Analysis of Synaptophysin-Positive Gastric Cancer Patients: A Retrospective Study
Funding: The authors received no specific funding for this work.
Xuemei Wei and Xu Zhang contributed equally to this work and shared the co-first authorship.
ABSTRACT
Background
This study aims to determine prognostic indicators of synaptophysin (SYN) positive gastric cancer with neuroendocrine type by analyzing differences in serological indicators and survival between SYN-positive and SYN-negative gastric cancer patients and to provide a theoretical basis for the prognosis of patients.
Methods
The medical records of 1298 gastric cancer patients who had received surgical treatment between January 2019 and December 2021 at The First Medical Center of Chinese PLA General Hospital were assessed, and 59 patients were enrolled in this study, to analyze serological indices and survival differences between patients with SYN-positive and SYN-negative gastric cancer.
Results
There were statistically significant differences between the expression of SYN and genetic history, tumor differentiation degree, and Lauren's typing (p = 0.036, 0.040, and 0.017, respectively). The level of neuron-specific enolase in SYN-positive patients was higher than that in SYN-negative patients (p = 0.027). The level of hemoglobin (Hb) and mean corpuscular hemoglobin (MCH) was lower in SYN-positive patients when compared with SYN-negative patients (p = 0.023 and 0.019, respectively). The survival time of SYN-positive gastric cancer patients was statistically significantly different from that of SYN-negative gastric cancer patients (p = 0.0255).
Conclusions
SYN expression in patients with gastric cancer is correlated with the degree of differentiation and Lauren's typing. The prognosis of SYN-positive gastric cancer patients is worse than that of SYN-negative patients.
Abbreviations
-
- AFP
-
- alpha fetal protein
-
- AJCC
-
- American Joint Committee on Cancer
-
- ALB
-
- albumin
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BUN
-
- blood urea nitrogen
-
- CA125
-
- carbohydrate antigen 125
-
- CA15-3
-
- carbohydrate antigen 15-3
-
- CA19-9
-
- carbohydrate antigen 19-9
-
- CA724
-
- carbohydrate antigen 724
-
- CEA
-
- carcinoembryonic antigen
-
- CgA
-
- chromogranin A
-
- CK
-
- creatine kinase
-
- Cr
-
- creatinine
-
- CYFRA21-1
-
- cytokeratin fragment antigen 21-1
-
- DAB
-
- diaminobenzidine
-
- DB
-
- direct bilirubin
-
- DCLK1
-
- doublecortin, like kinase 1
-
- GC-NEF
-
- gastric cancer with neuroendocrine features
-
- GGT
-
- γ-glutamyl transpeptidase
-
- GLU
-
- glucose
-
- Hb
-
- hemoglobin
-
- HCT
-
- hematocrit
-
- IHC
-
- immunohistochemistry
-
- LDH
-
- lactate dehydrogenase
-
- MCH
-
- mean corpuscular hemoglobin
-
- MCHC
-
- mean corpuscular hemoglobin concentration
-
- MCV
-
- mean corpuscular volume
-
- NEC
-
- neuroendocrine carcinoma
-
- NSE
-
- neuron-specific enolase
-
- PG I
-
- pepsinogen I
-
- PG II
-
- pepsinogen II
-
- PLT
-
- platelet
-
- RBC
-
- red blood cell
-
- RDW
-
- red blood cell distribution width
-
- SCC
-
- squamous cell carcinoma antigen
-
- SYN
-
- synaptophysin
-
- TB
-
- total bilirubin
-
- TNM
-
- tumor-node-metastasis
-
- TP
-
- total protein
-
- UA
-
- uric acid
-
- WBC
-
- white blood cell
-
- WHO
-
- World Health Organization
1 Introduction
Gastric cancer ranks among the top five cancers globally in terms of incidence and mortality. In China, it is the third most common cancer and the third leading cause of cancer-related deaths [1, 2]. Gastric cancer with neuroendocrine features (GC-NEF) is a rare subtype, accounting for only 0.1%–0.6% of all gastric cancers. This type of gastric cancer is characterized by rapid growth, frequent lymphovascular invasion, and a high metastatic rate, exhibiting aggressive biological behavior [3, 4]. The symptoms of GC-NEF are nonspecific, making it difficult to distinguish it from other types of gastric cancer through imaging and endoscopy. Most cases are diagnosed only after the development of lymph node metastases or distant metastases, resulting in poor prognoses, and short survival times for GC-NEF patients. Due to its low incidence and heterogeneity, our understanding of GC-NEF remains limited. Currently, the diagnosis of GC-NEF relies on the immunohistochemical (IHC) detection of chromogranin A (CgA) and synaptophysin (SYN) expression [5, 6].
Many studies on GC-NEF have focused on treatment and prognosis, with few studies examining SYN expression alone [4, 7, 8]. This study aimed to analyze the relationship between SYN expression and hematological indicators and any correlation in prognosis. Therefore, CgA expression in GC-NEF was excluded to minimize the interference of other neuroendocrine factors. By analyzing the serological indices and survival differences between SYN-positive and SYN-negative gastric cancer patients, we aimed to identify valuable laboratory markers for predicting SYN-positive GC-NEF and to provide a theoretical basis for patient prognosis.
2 Materials and Methods
2.1 Materials
From 1298 gastric cancer patients who underwent surgical treatment at The First Medical Center of Chinese PLA General Hospital between January 2019 and December 2021, 59 GC-NEF patients were identified. Clinical data were collected, and survival rates were determined. The follow-up period was defined as the interval between diagnosis and the last follow-up, which was the date of death for those patients who had died. As this study was a retrospective analysis, some data were missing.
The histopathological features of the tumor specimens were classified according to the WHO criteria [9, 10]. The TNM staging was determined based on the 7th Edition of the American Joint Committee on Cancer guidelines (2010) for neuroendocrine carcinoma (NEC). According to the above criteria, the degree of differentiation of gastric cancer includes well-differentiated, moderately differentiated, and poorly differentiated. Well-differentiated: Tumor cells resemble normal gastric gland cells, exhibiting a relatively intact structure, small nuclei, and fewer mitotic figures. Moderately differentiated: The characteristics of tumor cells are intermediate between well-differentiated and poorly differentiated, with significant changes in cell morphology and structure while retaining some glandular architecture. Poorly differentiated: Tumor cells exhibit substantial differences from normal cells, with irregular cell morphology, numerous mitotic figures, and nearly complete loss of glandular structure.
A retrospective analysis was conducted to summarize the clinical and pathological characteristics of patients with gastric cancer and to analyze factors affecting prognosis. The inclusion criteria for cases were as follows, (1) Newly diagnosed patients receiving inpatient treatment at The First Medical Center of Chinese PLA General Hospital with pathologically confirmed gastric cancer with neuroendocrine differentiation; (2) IHC results of the patients contained SYN and CgA, with CgA being negative; (3) All patients had normal organ function and no serious underlying diseases, including conditions that could significantly impact biochemical and hematological parameters, such as hepatic and renal dysfunction, hematological disorders, and cardiovascular diseases; (4) Comprehensive and detailed medical records. Patients were excluded if they had other malignant tumors, severe chronic diseases, or simple neuroendocrine tumors.
This study was approved by the Ethics Committee of The First Medical Center of Chinese PLA General Hospital (Approval Number: S2021-129-01). Since this study uses the medical records obtained from previous clinical diagnoses and treatments, and the privacy and personal information of the subjects are protected, the ethics committee has approved the waiver of informed consent.
2.2 Methods
Biochemical and tumor marker results were measured using the Cobas8000 c701 and Cobas8000 c602 instruments (Roche Diagnostics, Mannheim, Germany). Blood routine results were measured using the SYSMEX XN9000 (Sysmex Corporation, Kobe, Japan) using the original reagents provided with the instrument. After testing, the specimens were stored between 2°C and 8°C. Quality control procedures were conducted on the day of testing to ensure the accuracy and reliability of the results. In this study, IHC staining was performed to detect the neuroendocrine biomarkers SYN and CgA in gastric cancer tissues. After the standard procedure of deparaffinization and rehydration, 4-μm tissue sections underwent epitope retrieval using a citrate buffer (pH 6.0) at 120°C for 4 min in a pressure cooker. Endogenous peroxidase activity was inhibited by treating the sections with 3% hydrogen peroxide at room temperature for 15 min before applying the primary antibodies. To prevent nonspecific binding, the slides were incubated with goat serum at room temperature for 30 min. The sections were then exposed to anti-DCLK1 (1:700, ab109029; Abcam, Cambridge, MA) and kept overnight at 4°C. After washing with PBS and distilled water, the sections were incubated with an anti-rabbit secondary antibody (PV-6001, ZSGB-BIO, Beijing, China) at 37°C for 30 min. The antibody reaction was visualized using diaminobenzidine (DAB, 1∶20, ZLI-9017, ZSGB-BIO, Beijing, China). For the staining of SYN and CgA, anti-SYN (1∶500; ab32127; Abcam, Cambridge, MA) and anti-CgA (1∶500; ab15160; Abcam, Cambridge, MA) antibodies were utilized. The slides were then analyzed under a Nikon 80i microscope (Nikon Corporation, Tokyo, Japan) equipped with a DXM1200C camera for bright field microscopy [11]. SYN results were evaluated by two independent pathologists, and a three-level review system was implemented to ensure accuracy and precision.
In this study, all patients were classified into two groups based on SYN expression: negative and positive. The following five aspects were analyzed: (1) Basic data of gastric cancer patients grouped by SYN positivity or negativity; (2) The relationship between serological indices and the presence of SYN-positive gastric cancer; (3) A correlation analysis to determine any relationship between SYN expression and statistically significant indicators in the two groups; (4) A comparative analysis to ascertain survival differences between the SYN-positive and SYN-negative groups; (5) A comparison of survival times of patients with different pathological types, TNM stages, and degrees of differentiation.
2.3 Statistical Analysis
Statistical analysis was performed using IBM SPSS 22.0 (IBM Corp., Armonk, NY, USA), and graphs were created with GraphPad Prism 9.5 (GraphPad Software, San Diego, CA, USA). Qualitative data were expressed as positive rates, and the Chi-square test was used for statistical analysis. All quantitative data in this study were skewed and represented by median (P25, P75). Comparisons between groups were performed using the Wilcoxon rank sum test, and Spearman correlation analysis was used for correlations. Survival estimates were performed using the Kaplan–Meier method in conjunction with the Log-rank test, with p < 0.05 considered statistically significant.
3 Results
3.1 Clinicopathological Characteristics
A total of 59 patients with gastric cancer were included in the study. Detailed information is presented in Table 1. Among the cases, 35 were positive for SYN, 24 were negative for SYN, and six had a family history. According to the Lauren classification, the ratio of intestinal-type to diffuse-type plus mixed-type cases was 14:42. Additionally, 29 cases exhibited TNM stage I + II, while 30 cases demonstrated TNM stage III + IV. There was a significant correlation between SYN expression and genetic history, tumor differentiation degree, and Lauren typing (p = 0.036, 0.040, and 0.017, respectively).
| Variables | Features | SYN | Z/χ2 | p | |
|---|---|---|---|---|---|
| Positive n (%) or median [P25, P75] | Negative n (%) or median [P25, P75] | ||||
| Gender | Male | 29 (63.0) | 17 (37.0) | 1.198 | 0.218 |
| Female | 6 (46.2) | 7 (53.8) | |||
| Age (year) | 65 [60, 69] | 63 [57, 72] | −0.031 | 0.975 | |
| Systolic blood pressure (mmHg) | 130 [114, 143] | 128 [122, 146] | −0.309 | 0.757 | |
| Diastolic blood pressure (mmHg) | 78 [69, 87] | 80 [73, 89] | −1.430 | 0.153 | |
| BMI (kg/m2) | 24.4 [21.6, 26.8] | 22.4 [21.2, 27.5] | −0.494 | 0.621 | |
| Smoking history | Yes | 18 (60.0) | 12 (40.0) | 0.012 | 0.562 |
| No | 17 (58.6) | 12 (41.4) | |||
| Drinking history | Yes | 18 (66.7) | 9 (33.3) | 1.113 | 0.215 |
| No | 17 (53.1) | 15 (46.9) | |||
| Genetic history | Yes | 6 (100.0) | 0 (0) | 4.58 | 0.036 |
| No | 29 (54.7) | 24 (45.3) | |||
| Borrmann | Nodular | 9 (56.3) | 7 (43.8) | 0.272 | 0.965 |
| Restricted ulcer | 16 (57.1) | 12 (42.9) | |||
| Infiltrating ulcer | 5 (62.5) | 3 (37.5) | |||
| Diffuse infiltration | 4 (66.7) | 2 (33.3) | |||
| Position | Cardia, fundus of stomach | 7 (63.6) | 4 (36.4) | 5.86 | 0.119 |
| Gastric curvature, angle | 7 (38.9) | 11 (61.1) | |||
| Gastric body, greater curvature | 12 (80.0) | 3 (20.0) | |||
| Pylorus, gastric antrum | 9 (60.0) | 6 (40.0) | |||
| Differentiation degree | High to moderately differentiated adenocarcinoma | 5 (83.3) | 1 (16.7) | 8.311 | 0.04 |
| Moderately to poorly differentiated adenocarcinoma | 13 (54.2) | 11 (45.8) | |||
| Poorly differentiated adenocarcinoma—signet ring cell carcinoma | 17 (68.0) | 8 (32.0) | |||
| Other | 0 (0) | 4 (100.0) | |||
| Lauren classification | Intestinal pattern | 12 (85.7) | 2 (14.3) | 5.585 | 0.017 |
| Diffuse type + mixed type | 22 (52.4) | 20 (47.6) | |||
| Stage | I + II | 16 (55.2) | 13 (44.8) | 0.407 | 0.355 |
| III + IV | 19 (63.3) | 11 (36.7) | |||
| Tumor location (T) | I | 10 (66.7) | 5 (33.3) | 2.266 | 0.322 |
| II | 0 (0) | 0 (0) | |||
| III | 15 (50.0) | 15 (50.0) | |||
| IV | 10 (71.4) | 4 (28.6) | |||
| Lymph node (N) | Yes | 11 (64.7) | 6 (35.3) | 0.287 | 0.407 |
| No | 24 (57.1) | 18 (42.9) | |||
| Metastasis (M) | Yes | 25 (55.6) | 20 (44.4) | 1.115 | 0.23 |
| No | 10 (71.4) | 4 (28.6) | |||
| Tumor number | Single | 30 (56.6) | 23 (43.4) | 1.031 | 0.304 |
| Multiple | 4 (80.0) | 1 (20.0) | |||
| Tumor size | Diameter < 3 cm | 12 (57.1) | 9 (42.9) | 0.027 | 0.608 |
| Diameter ≥ 3 cm | 20 (57.1) | 15 (42.9) | |||
- Note: As this study was a retrospective analysis, some data were missing.
- Abbreviations: BMI, body mass index; SYN, synaptophysin.
3.2 Differences in Serological Indices Between SYN-Positive and SYN-Negative Gastric Cancer Patients
The level of neuron-specific enolase (NSE) in SYN-positive patients was higher than in SYN-negative patients (Z = −2.207, p = 0.027). Conversely, the levels of Hb and MCH were found to be lower in SYN-positive patients than in SYN-negative patients (Z = −2.187, −2.354; p = 0.023, 0.019, respectively). The remaining indicators showed no statistically significant differences (Table 2).
| Variables | SYN negative median [P25, P75] | SYN positive median [P25, P75] | Z | p |
|---|---|---|---|---|
| ALT (U/L) | 13.1 [9.7, 20.5] | 11.6 [6.6, 16.9] | −0.363 | 0.717 |
| AST (U/L) | 15.7 [14.3, 21.5] | 13.9 [11.6, 16.9] | −0.532 | 0.594 |
| TP (g/L) | 66.3 [63.0, 71.7] | 63.9 [58.2, 67.7] | −0.185 | 0.853 |
| ALB (g/L) | 39.7 [35.2, 40.5] | 37.2 [34.1, 42.2] | −0.355 | 0.723 |
| TB (μmol/L) | 9.8 [8.6, 14.7] | 8.8 [5.2, 13.4] | −1.420 | 0.156 |
| DB (μmol/L) | 3.5 [2.6, 4.8] | 3.2 [2.3, 3.5] | −1.305 | 0.192 |
| ALP (U/L) | 78.2 [50.5, 86.8] | 69.0 [59.7, 81.2] | −0.126 | 0.899 |
| GGT (U/L) | 20.7 [13.7, 42.0] | 17.4 [12.9, 23.4] | −0.624 | 0.533 |
| GLU (mmol/L) | 5.18 [4.79, 5.95] | 5.02 [4.53, 5.70] | −0.918 | 0.358 |
| BUN (μmol/L) | 4.94 [4.20, 6.36] | 4.72 [3.35, 5.72] | −0.093 | 0.926 |
| Cr (μmol/L) | 78.2 [69.5, 89.0] | 66.0 [56.8, 80.1] | −1.459 | 0.145 |
| UA (μmol/L) | 278.8 [242.2, 334.6] | 261.0 [154.3, 363.3] | −0.872 | 0.383 |
| CK (U/L) | 57.3 [47.0, 96.7] | 44.1 [39.8, 75.4] | −0.687 | 0.492 |
| LDH (U/L) | 151.2 [126.1, 170.4] | 152.3 [130.9, 181.7] | −0.889 | 0.374 |
| Ca2+ (mmol/L) | 2.27 [2.14, 2.37] | 2.23 [2.16, 2.31] | −0.422 | 0.673 |
| Phosphorus (mmol/L) | 1.13 [0.96, 1.25] | 1.19 [1.01, 1.25] | −0.692 | 0.489 |
| Mg2+ (mmol/L) | 0.93 [0.84, 0.95] | 0.89 [0.83, 0.92] | −1.449 | 0.147 |
| K+ (mmol/L) | 4.02 [3.82, 4.26] | 4.08 [3.80, 4.30] | −0.031 | 0.975 |
| Na+ (mmol/L) | 140.5 [136.0, 142.7] | 139.7 [137.6, 141.9] | −0.208 | 0.835 |
| Cl− (mmol/L) | 102.7 [101.3, 105.4] | 103.8 [102.6, 105.3] | −1.327 | 0.184 |
| CEA (μg/L) | 2.29 [1.25, 3.87] | 2.50 [1.78, 4.44] | −0.424 | 0.671 |
| AFP (μg/L) | 2.88 [2.13, 3.95] | 2.34 [1.92, 3.18] | −0.941 | 0.347 |
| CA125 (U/mL) | 11.84 [8.41, 21.01] | 12.42 [7.98, 19.00] | −0.648 | 0.517 |
| Ca19-9 (U/mL) | 11.68 [5.26, 23.54] | 10.04 [7.25, 17.74] | −0.278 | 0.781 |
| CA15-3 (U/mL) | 7.46 [5.73, 10.54] | 5.00 [4.12, 7.80] | −2.145 | 0.032 |
| CA724 (U/mL) | 3.32 [1.57, 7.13] | 1.63 [0.99, 9.80] | −0.640 | 0.522 |
| CYFRA21-1 (ng/mL) | 3.37 [2.43, 4.26] | 2.38 [2.06, 6.78] | −2.276 | 0.023 |
| NSE (ng/mL) | 11.85 [9.04, 14.90] | 12.66 [9.84, 24.43] | −2.207 | 0.027 |
| SCC (ng/mL) | 1.2 [0.7, 1.6] | 0.9 [0.7, 1.1] | −1.258 | 0.208 |
| PG I (ng/mL) | 94.5 [38.9, 117.0] | 52.3 [40.0, 90.4] | −1.385 | 0.166 |
| PG II (ng/mL) | 18.8 [11.5, 26.4] | 14.6 [6.8, 25.2] | −1.058 | 0.290 |
| PG I/II | 4.4 [2.8, 6.0] | 4.5 [2.4, 6.3] | −0.180 | 0.857 |
| Hb (g/L) | 127 [120, 145] | 115 [94, 131] | −2.187 | 0.023 |
| RBC (× 1012/L) | 4.31 [3.97, 4.60] | 3.91 [3.53, 4.72] | −1.391 | 0.164 |
| WBC (× 109/L) | 6.11 [4.48, 7.03] | 5.61 [4.67, 7.58] | −0.135 | 0.892 |
| Neutrophil | 0.610 [0.528, 0.712] | 0.630 [0.505, 0.740] | −0.477 | 0.633 |
| Lymphocyte | 0.266 [0.174, 0.367] | 0.274 [0.185, 0.389] | −0.469 | 0.639 |
| Monocyte | 0.078 [0.058, 0.091] | 0.065 [0.061, 0.090] | −0.278 | 0.781 |
| HCT | 0.379 [0.361, 0.412] | 0.326 [0.292, 0.415] | −1.781 | 0.075 |
| MCV (L/L) | 87.8 [83.3, 92.5] | 87.6 [80.4, 88.8] | −0.954 | 0.340 |
| MCH (pg) | 30.4 [28.6, 32.0] | 28.9 [25.1, 30.2] | −2.354 | 0.019 |
| MCHC (g/L) | 341 [326, 346] | 339 [316, 346] | −1.408 | 0.159 |
| RDW (%) | 12.5 [12.1, 13.3] | 13.9 [12.6, 18.0] | −2.506 | 0.012 |
| PLT (× 109/L) | 210 [154, 284] | 224 [186, 293] | −0.262 | 0.793 |
- Abbreviations: AFP, alpha fetal protein; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CA125, carbohydrate antigen 125; CA15-3, carbohydrate antigen 15-3; CA19-9, carbohydrate antigen 19-9; Ca2+, calcium; CA724, carbohydrate antigen 724; CEA, carcinoembryonic antigen; Cl−, chlorine; CK, creatine kinase; Cr, creatinine; CYFRA21-1, cytokeratin fragment antigen 21-1; DB, direct bilirubin; FEER, ferritin; GGT, γ-glutamyl transpeptidase; GLU, glucose; Hb, hemoglobin; HCG, human chorionic gonadotropin; HCT, hematocrit; K+, potassium; LDH, lactate dehydrogenase; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean red blood cell volume; Mg2+, magnesium; Na+, sodium; NSE, neuron-specific enolase; PG I, pepsinogen I; PG II, pepsinogen II; PLT, platelet; RBC, red blood cell; RDW, red blood cell distribution width; SCC, squamous cell carcinoma antigen; TB, total bilirubin; TP, total protein; UA, uric acid; WBC, white blood cell.
3.3 Correlation Analysis Among Variables in Patients With Gastric Cancer
A significant correlation was observed between SYN and several other variables, including genetic history, Lauren typing, carbohydrate antigen 15–3 (CA 15–3), cytokeratin fragment antigen (CYFRA21-1), NSE, Hb, MCH, and red blood cell distribution width (RDW) (p < 0.05). Significant correlations were also noted between CYFRA21-1/genetic history, NSE/genetic history, NSE/differentiation degree, MCH/CA15-3, Hb/MCH, and Hb/RDW (p < 0.01) (Table 3).
| SYN | Genetic history | Differentiation | Lauren classification | Ca15-3 | CYFRA21-1 | NSE | Hb | MCH | RDW | |
|---|---|---|---|---|---|---|---|---|---|---|
| SYN | 1.000 | |||||||||
| Genetic history | −0.279a | 1.000 | ||||||||
| Differentiation | −0.173 | 0.223 | 1.000 | |||||||
| Lauren classification | 0.310a | −0.205 | 0.074 | 1.000 | ||||||
| Ca15-3 | −0.282a | 0.250 | −0.034 | −0.063 | 1.000 | |||||
| CYFRA21-1 | −0.299a | 0.359b | 0.089 | −0.221 | 0.238 | 1.000 | ||||
| NSE | 0.290a | −0.356b | −0.398b | −0.007 | −0.145 | −0.300a | 1.000 | |||
| Hb | −0.290a | 0.110 | −0.104 | −0.186 | 0.174 | 0.063 | 0.025 | 1.000 | ||
| MCH | −0.312a | 0.198 | 0.004 | −0.160 | 0.491b | 0.049 | −0.065 | 0.593b | 1.000 | |
| RDW | 0.332a | −0.049 | −0.211 | 0.113 | −0.205 | −0.173 | 0.199 | −0.601b | −0.531b | 1.000 |
- Abbreviations: CA15-3, carbohydrate antigen 15-3; CYFRA21-1, cytokeratin fragment antigen 21-1; Hb, hemoglobin; MCH, mean corpuscular hemoglobin; NSE, neuron-specific enolase; RDW, red blood cell distribution width; SYN, synaptophysin.
- a Indicates a significant correlation at the 0.05 level (both sides).
- b Indicates a significant association at the 0.01 level (bilateral).
3.4 Survival Analysis of SYN-Positive Patients
A total of 28 patients died during the study, and all patients were followed up. The 1-, 2-, and 3-year survival rates for patients with SYN-positive gastric cancer were 95.8%, 56.8%, and 19.0%, respectively. In comparison, the 1-, 2-year, and 3-year survival rates for patients with SYN-negative gastric cancer were 88.6%, 75.3%, and 49.6%, respectively. These results exhibit a statistically significant difference in survival curves (p = 0.0255), as shown in Figure 1.
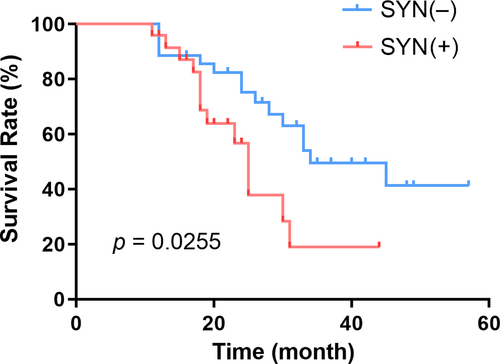
Survival curves of SYN-positive and negative patients. The 1-, 2-, and 3-year survival rates of SYN-positive and SYN-negative gastric cancer patients were statistically different (p < 0.05). SYN, synaptophysin.
3.5 Survival Analysis of Gastric Cancer Patients With Different Degrees of Differentiation, TNM Staging, and Lauren's Classification
The 1-, 2-, and 3-year survival rates were 93.3%, 71.6%, and 55.4% in patients with well-, moderately, and poorly differentiated adenocarcinoma, respectively. In contrast, the 1-, 2-, and 3-year survival rates in patients with signet ring cell carcinoma were 88.0%, 62.7%, and 23.1%, respectively, However, there was no statistically significant difference in the 1-, 2-, and 3-year survival rates among patients with well, moderately, and poorly differentiated adenocarcinoma, and those with signet ring cell carcinoma (p = 0.1597) (Figure 2a). The 1-, 2-, and 3-year survival rates for patients with TNM stage I + II were 100%, 87.6%, and 64.8%, respectively. The 1-, 2-, and 3-year survival rates for patients with TNM stage III + IV were 82.8%, 50.4%, and 14.2%, respectively. Statistically significant differences were observed in the survival curves (p < 0.0001) (Figure 2b). In Lauren's classification, the 1-, 2-, and 3-year survival rates of patients with intestinal type were 100%, 83.1%, and 73.8%, respectively. In comparison, the 1-, 2-, and 3-year survival rates for patients with diffuse and mixed types were 89.2%, 67.3%, and 30.0%, respectively. The 1-, 2-, and 3-year survival rates for the remaining patients were 87.5%, 46.9%, and 0%, respectively. The survival curves were statistically significantly different (p = 0.0164), as illustrated in Figure 2c.
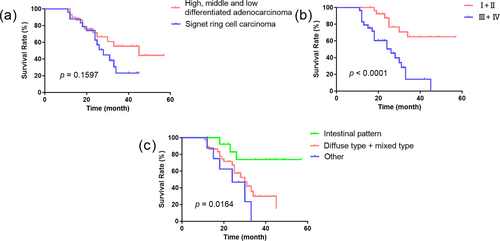
Survival curves of patients with different degrees of differentiation, TNM stages and Lauren’s classification. (a) Survival curves of different Lauren classifications. (b) Survival curves of different TNM stages. (c) Survival curves of different degrees of differentiation. TNM, tumor-node-metastasis.
4 Discussion
The objective of this study was to identify valuable laboratory markers for the diagnosis of GC-NEF. To this end, we analyzed the differences in serological markers and survival times between SYN-positive and SYN-negative gastric cancer patients. Furthermore, we sought to determine whether SYN expression could be a prognostic indicator of GC-NEF.
The degree of differentiation of gastric cancer is a key determinant of prognosis. Generally, a lower degree of differentiation is associated with a worse prognosis. According to the study by Zhang et al., patients with poorly differentiated NEC accompanied by distant metastasis have a poor prognosis [12]. Another study indicated that gastric cancer patients with a neuroendocrine differentiation ratio greater than 10% have a worse survival prognosis [13]. In our study, a comparison of the basic information from patients in the SYN-negative and SYN-positive groups revealed a significantly higher proportion of well-differentiated patients in the SYN-negative group. Additionally, survival curve analyses showed that the 1-, 2-, and 3-year survival rates of well-, moderately, and poorly differentiated adenocarcinoma were significantly higher than those of signet-ring cell carcinoma (Figure 2a), consistent with previous findings. The survival rates of patients with early and intermediate TNM stages were significantly higher than those of patients with advanced stages (Figure 2b), indicating that the overall survival results of our data align with the current prognostic understanding of gastric cancer.
SYN is a neuroendocrine marker, a clear vesicle-like integrated membrane protein widely present in neuroendocrine cells and located on the synaptic vesicles of neurons. In cases where the tumor exhibits neuroendocrine hyperplasia, SYN expression may become diffusely positive. Gastric neuroendocrine tumors can be diagnosed based on SYN expression [14, 15]. Previous studies have indicated that neuroendocrine tumors generally have a poor prognosis, which aligns with our findings that highly differentiated adenocarcinoma constitutes a low proportion of SYN-positive patients [16-18]. In our study, other types of gastric cancers, including mucinous adenocarcinoma and simple neuroendocrine tumors, were found to have less favorable prognoses than common adenocarcinoma. These results demonstrate that the proportion of patients in the SYN-positive group was significantly higher than that in the negative group, which is consistent with our initial hypothesis. Notably, no significant difference was found between poorly differentiated adenocarcinoma and signet ring cell carcinoma, challenging previous views on this subject. One potential explanation is that the underlying mechanisms and specific etiologies of neuroendocrine tumors remain poorly understood [19, 20], particularly considering the limited sample size of highly differentiated adenocarcinoma and other adenocarcinoma types in this study.
Lauren's classification divides gastric cancer into three categories: intestinal type, diffuse type, and mixed type. The intestinal type is associated with a more favorable prognosis, while the other two categories are linked to relatively poor prognoses [21-23]. This is consistent with our research, and in our research, the proportion of intestinal gastric cancer in the SYN-negative group was significantly higher than that in the positive group, and the survival curve demonstrated a higher survival rate for the intestinal type (Figure 2c). In the SYN-positive group, a higher proportion of mixed and diffuse types with poor prognoses was observed, indicating that gastric cancer associated with neuroendocrine factors tends to have a relatively poor prognosis. The results also reveal a correlation with genetic factors, with the negative group exhibiting a stronger correlation with genetic predisposition than the positive group. This suggests that individuals with a family history of genetic predisposition may be more susceptible to developing common gastric cancer.
In this study, we compared serological variables between the SYN-negative and SYN-positive groups and found that NSE levels were significantly higher in the SYN-positive group. CA 15–3 and CYFRA21-1 levels were lower in the positive group, but no statistically significant differences were identified in the biochemical parameters. NSE is a specific enzyme predominantly localized within the cytoplasm of neurons and neuroendocrine cells, which is released into the blood during neuronal edema and degeneration. Consequently, the presence of neuroendocrine tumors is accompanied by a notable increase in this enzyme [24, 25]. Most other tumor markers exhibited a significant decline, with PG-I, PG-II, CA724, and other indicators closely associated with gastric cancer showing particularly pronounced reductions. This may be attributed to the neuroendocrine phenomena in tumors, which dominate tumor development. Consequently, the secretion of common gastric cancer tumor markers is diminished, resulting in elevated NSE levels and reduced levels of most other tumor markers [26, 27]. Given that neuroendocrine gastric cancer has a worse prognosis and more rapid disease progression, our analysis of routine blood tests indicated significant reductions in HB and MCH levels in the positive group [28]. Cancer can lead to the loss of beneficial substances, including proteins and sugars, resulting in anemia and malnutrition [29]. In our study, both Hb and MCH levels exhibited declines in the positive group. The decrease in Hb led to variations in red blood cell volume, resulting in a significant increase in RDW in the positive group. Correlation analysis confirmed the association between SYN and significant indicators, thereby substantiating the link between SYN and neuroendocrine gastric cancer.
In this study, all patients in the SYN-positive and SYN-negative groups were monitored throughout the follow-up period. The 1-year survival rate of the negative group was 88.6%, slightly higher than the results of similar studies in the literature. However, the 3-year survival rate of 49.6% aligns with findings from the majority of similar studies [30-33]. The 1-year survival rate of 95.8% observed in the positive group was higher than the results reported in some studies. However, the 3-year survival rate of 19.0% was significantly lower than the average reported in the literature. Despite most current studies indicating a poorer prognosis for neuroendocrine tumors, there remains no consensus, with some studies showing minimal correlation between neuroendocrine tumors and survival [34]. The discrepancies in these findings may be attributable to several factors, including differences in sample size, unclear follow-up periods, inconsistencies in medication regimens, and the complexity of underlying pathogenesis.
While the majority of indicators for SYN-positive gastric cancer patients were analyzed according to established criteria, the low prevalence of the disease in this study resulted in a relatively small number of cases drawn from a single center, representing a limitation of the study. In future phases, we will expand the sample size (a multicenter study) and extend the follow-up period to enhance the robustness of our research.
5 Conclusions
The present study revealed a correlation between SYN expression in gastric cancer patients and the degree of differentiation and Lauren's classification. In patients with SYN-positive gastric cancer, there was a notable increase in NSE levels and a corresponding decrease in Hb levels, which were associated with a relatively poor survival prognosis.
Author Contributions
Xuemei Wei: investigation (equal), writing – original draft (equal). Xu Zhang: formal analysis (equal), investigation (equal), software (equal). Jiaqi Zhang: data curation (equal), formal analysis (equal). Lin Zhu: formal analysis (equal), validation (equal). Guanghong Guo: project administration (equal), writing – review and editing (equal).
Acknowledgments
We thank all the colleagues in the Department of Clinical Laboratory of the First Medical Center of Chinese PLA General Hospital for their support in this study.
Ethics Statement
This study involving human participants was reviewed and approved by The First Medical Center of Chinese PLA General Hospital (approval number: S2021-129-01).
Consent
Since this study uses the medical records obtained from previous clinical diagnoses and treatments, and the privacy and personal information of the subjects are protected, the ethics committee has approved the waiver of informed consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.




