Clinical utility of six serum tumor markers for the diagnosis of lung cancer
Abstract
Background
With the increasing prevalence of lung cancer, it has become imperative to identify reliable biomarkers that can aid in early detection and prognosis assessment. Therefore, we sought to investigate the potential utility of six serum tumor markers as diagnostic and prognostic tools for lung cancer patients. By analyzing a large cohort of patients with different stages and subtypes of lung cancer, we hoped to shed light on the predictive value and accuracy of each marker individually, as well as their combined performance. This study should not only provide valuable insights into the biology and pathogenesis of lung cancer but also pave the way for personalized treatment strategies based on individual patient profiles.
Methods
The serum levels of the tumor markers progastrin-releasing peptide (ProGRP), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cytokeratin 19 fragment (CYFRA21-1), carbohydrate antigen 19-9 (CA19-9) and squamous cell carcinoma antigen (SCCA) were meticulously assessed in a cohort comprising 324 individuals diagnosed with lung cancer and an additional 51 patients with benign lung disease. The measurements were conducted using cutting-edge techniques such as ELISA, electrochemical luminescence, and chemiluminescence methods. Differences between groups and the impact of these markers on lung cancer diagnosis were analyzed.
Results
The serum levels of ProGRP, NSE, and CEA were significantly higher in lung cancer patients than in patients with benign lung disease (p < 0.01). NSE had the highest sensitivity for squamous cell carcinomas (SC), while CEA had the highest sensitivity for adenocarcinomas (AC). ProGRP and NSE had higher sensitivities than other markers for small cell carcinomas (SCC). Combining the six tumor markers resulted in higher sensitivities for SC (70.6%), AC (77.4%), and SCC (80%) compared with any single test. Receiver operator characteristic analysis showed that ProGRP and NSE had a greater area under the curve (AUC) in SCC (0.886 and 0.775) than SC and AC, while CEA had a higher AUC in AC (0.716), and NSE had a higher AUC than other markers in SC (0.719).
Conclusions
ProGRP and NSE are effective serum tumor markers for SCC, whereas CEA and NSE may aid in the diagnosis of AC and SC. Combining the detection of ProGRP, NSE, CYFRA21-1, CEA, and SCCA significantly improves sensitivity when diagnosing lung cancer.
Abbreviations
-
- AC
-
- adenocarcinoma
-
- AUC
-
- area under the ROC curve
-
- CA19-9
-
- carbohydrate antigen 19-9
-
- CEA
-
- carcinoembryonic antigen
-
- CYFRA21-1
-
- cytokeratin 19 fragment
-
- NSCLC
-
- non-small cell lung cancer
-
- NSE
-
- neuron-specific enolase
-
- ProGRP
-
- progastrin-releasing peptide
-
- ROC
-
- receiver operating characteristic
-
- SC
-
- squamous cell carcinoma
-
- SCC
-
- small cell carcinomas
-
- SCCA
-
- squamous cell carcinoma antigen
1 INTRODUCTION
Pulmonary carcinoma is a prevalent malignancy worldwide, with its incidence and mortality rates increasing rapidly in many countries, particularly developed ones, over the past 50 years. Around 1.2 million individuals are reported to suffer from this debilitating disease each year. This illness has a devastating impact, with a large majority of patients, around 1.1 million, dying of lung cancer annually [1].
It is relatively easy to differentiate cancer patients from healthy individuals based on clinical manifestations, chest radiography, or laboratory examination. However, both lung cancer and benign disease present as lung shadows in chest imaging, making it challenging to distinguish between the two. Therefore, pathological diagnosis is often employed. We anticipated the existence of tumor markers that would be present at differing levels in benign lung disease and lung cancer and could serve as valuable tools for differential diagnosis among patients who are either financially constrained or unsuited to undergoing a lung puncture. We also expected to identify tumor markers capable of predicting the pathological type of lung cancer. Early detection of pulmonary carcinoma is crucial in clinical practice. Although tumor markers associated with pulmonary carcinoma have been identified, the quest for early diagnostic biomarkers for this disease remains elusive. As a result, patients with pulmonary carcinoma are usually diagnosed when the disease has already progressed to a later stage and experience poor treatment outcomes [2].
The association between the occurrence and progression of lung cancer has been reported for progastrin-releasing peptide (ProGRP), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), CYFRA21-1, CA19-9, and squamous cell carcinoma antigen (SCCA). This study investigated the clinical utility of the six serum tumor markers in differentiating between lung cancer and benign lung diseases through the comparative analysis of patient samples.
2 MATERIALS AND METHODS
2.1 Human subjects
All patients diagnosed with lung cancer and enrolled in this study received treatment at the Seventh Medical Center of Chinese PLA General Hospital. The study was approved by the hospital's ethical committee, and each subject provided consent for their blood sample to be taken. The pathological or cytological confirmation was obtained for all cases, including 68 cases of squamous cell carcinoma (SC), 186 cases of adenocarcinomas (AC), and 70 cases of small cell carcinomas (SCC). Patients diagnosed with pulmonary infection and chronic obstructive pulmonary disease, totaling 51 individuals, had previously been diagnosed with benign lung disease. Serum specimens were collected from each participant and preserved at 4 and −70°C for long-term storage.
2.2 Methods
Serum ProGRP was detected using the enzyme-linked immunosorbent assay method (CanAg ProGRP EIA, Fujirebio Diagnostics), while CEA, NSE, CYFRA21-1, and CA19-9 were detected using electrochemical luminescence methods (Roche Cobas e601, Roche Diagnostics). SCCA was detected using a chemiluminescence immunoassay (Magulumi 4000, Snibe Diagnostic). The critical values for ProGRP, CEA, NSE, CYFRA21-1, SCCA, and CA19-9 were 49 ng/mL, 4.6 ng/mL, 15.2 ng/mL, 3.3 ng/mL, 2.5 ng/mL, and 39 U/mL, respectively, which were determined according to the kit instructions. Positive tests were defined as results exceeding the aforementioned values. In combined detection analysis, a positive overall result was determined whether any marker tested positive; conversely, an overall negative result was established when all markers in the group tested negative.
2.3 Statistical analysis
The analysis was conducted using SPSS19.0 (SPSS Inc.). As the serum tumor marker data exhibited a skewed distribution, median and range were utilized to represent concentration and dispersion tendency, respectively. The nonparametric rank sum test was employed for the comparison of tumor marker levels between groups. A significance level of p < 0.05 was established, and receiver operator characteristic (ROC) curves were employed to determine the area under the curve (AUC), which was calculated to compare the diagnostic accuracy of the tested markers in predicting lung cancer pathological type.
3 RESULTS
3.1 Age, gender, and levels of six tumor markers in patients with pulmonary carcinoma versus those with benign lung disease
The disparities in age, gender, and serum concentrations of six tumor markers between the pulmonary carcinoma cohort and the benign lung disease group are summarized in Table 1. The number of male patients was twice that of female patients in the lung cancer group. No statistically significant differences were observed in the age distribution between patients with pulmonary carcinoma and those with benign lung disease. However, the serum levels of ProGRP, NSE, and CEA were found to be significantly elevated in patients with pulmonary carcinoma compared with those with a benign condition (p < 0.01).
| Group | n | Age (x ± s) | Gender, n (%) | ProGRP (ng/L) | NSE (ng/mL) | CYFRA21-1 (ng/mL) | CEA (ng/mL) | SCCA (ng/mL) | CA19-9 (U/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | M | R | M | R | M | R | M | R | M | R | M | R | |||
| Lung cancer | 324 | 61.11 ± 11.36 | 215 (66.36) | 109 (33.64) | 33.6a | 3187.9 | 13.3a | 321.6 | 2.8 | 499.9 | 4.0a | 2536.5 | 0.8 | 99.9 | 15.3 | 3434.4 |
| Lung benign disease | 51 | 69.10 ± 17.00 | 28 (54.90) | 23 (45.10) | 18.5 | 66 | 9.8 | 42.6 | 2.6 | 12.4 | 2.6 | 24.9 | 0.8 | 32.3 | 11.9 | 49.1 |
- Abbreviations: CA19-9, cytokeratin 19 fragment; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment; M, median; NSE, neuron-specific enolase; ProGRP, progastrin-releasing peptide; R, range; SCCA, squamous cell carcinoma antigen.
- a Pulmonary carcinoma compared with benign lung disease, p < 0.01.
3.2 Tumor marker serum levels in various types of pulmonary carcinoma
The serum levels of ProGRP, NSE, CA19-9, CEA, SCCA, and CYFRA21-1 in different pathological types of pulmonary carcinoma are summarized in Table 2. Patients diagnosed with SCC exhibited significantly elevated serum levels of ProGRP and NSE, in comparison with those diagnosed with SC or AC (p < 0.05). Conversely, patients with AC had significantly higher serum levels of CEA and CA19-9 than those with SC or SCC (p < 0.05). The serum levels of SCCA were found to be significantly higher in patients diagnosed with SC than in patients with AC or SCC (p < 0.05). The serum levels of CYFRA21-1 were found to be elevated in SC patients, compared with levels in SCC patients (p < 0.05).
| n | Median | Range | Z1 a | P1 a | Z2 b | P2 b | Z3 c | P3 c | |
|---|---|---|---|---|---|---|---|---|---|
| GRP (ng/L) | |||||||||
| Squamous Carcinoma | 68 | 32.5 | 633.9 | −0.40 | >0.05 | ||||
| Adenocarcinoma | 186 | 27 | 152.9 | −7.52 | <0.05 | ||||
| Small cell carcinoma | 70 | 105.3 | 3185.2 | −5.95 | <0.05 | ||||
| NSE (ng/mL) | |||||||||
| Squamous Carcinoma | 68 | 13.2 | 28.3 | −0.54 | >0.05 | ||||
| Adenocarcinoma | 186 | 12.8 | 73.3 | −3.47 | <0.05 | ||||
| Small Cell carcinoma | 70 | 15.8 | 321.6 | −2.59 | <0.05 | ||||
| CYFRA21-1 (ng/mL) | |||||||||
| Squamous Carcinoma | 68 | 3.4 | 62.5 | −0.84 | >0.05 | ||||
| Adenocarcinoma | 186 | 2.8 | 499.3 | −1.04 | >0.05 | ||||
| Small cell carcinoma | 70 | 2.7 | 128.2 | −2.08 | <0.05 | ||||
| CEA (ng/mL) | |||||||||
| Squamous Carcinoma | 68 | 2.5 | 61.9 | −5.02 | <0.05 | ||||
| Adenocarcinoma | 186 | 6.3 | 2536.4 | −3.74 | <0.05 | ||||
| Small cell carcinoma | 70 | 2.7 | 173.4 | −1.18 | >0.05 | ||||
| SCCA (ng/mL) | |||||||||
| Squamous Carcinoma | 68 | 1.15 | 28 | −3.35 | <0.05 | ||||
| Adenocarcinoma | 186 | 0.7 | 99.9 | −0.84 | >0.05 | ||||
| Small cell carcinoma | 70 | 0.9 | 61.1 | −2.26 | <0.05 | ||||
| CA19-9 (U/mL) | |||||||||
| Squamous Carcinoma | 68 | 11.3 | 46.1 | −4.32 | <0.05 | ||||
| Adenocarcinoma | 186 | 18.2 | 3434.4 | −3.73 | <0.05 | ||||
| Small cell carcinoma | 70 | 10.2 | 999.4 | −0.21 | >0.05 | ||||
- Abbreviations: CA19-9, cytokeratin 19 fragment; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment; NSE, neuron-specific enolase; ProGRP, progastrin-releasing peptide; SCCA, squamous cell carcinoma antigen.
- a Squamous carcinoma compared with adenocarcinoma.
- b Adenocarcinoma compared with small cell carcinoma.
- c Small cell carcinoma compared with squamous carcinoma.
3.3 Sensitivity, accuracy, specificity, and Youden's index of the tumor markers in different types of pulmonary carcinoma
The sensitivity, specificity, and accuracy of the six serum tumor markers varied in their diagnostic performance for the different types of pulmonary carcinoma analyzed here. Each of the six serum tumor markers exhibited high specificity but low sensitivity. The ranking of accuracy, from high to low, was as follows: in SC, NSE > ProGRP > SCCA = CA19-9 > CEA > CYFRA21-1; in AC, CEA > CYFRA21-1 > CA19-9 > NSE > ProGRP > SCCA; and in SCC, ProGRP > NSE > CEA > CA19-9 > CYFRA21-1 > SCCA. The implementation of joint detection resulted in a significant improvement in the sensitivity of lung cancer screening. Using the combination Pro GRP + NSE + CYFRA21-1 + CEA + SCCA, the sensitivity in SC was enhanced to 70.6%. For AC, the combination of ProGRP + NSE + CYFRA21-1 + CEA + SCCA + CA19-9 demonstrated an increased sensitivity of 77.4%. Finally, in SCC, the combination of ProGRP + NSE + CYFRA21-1 + CEA resulted in increased sensitivity of up to 80.0% (Table 3).
| Markers | Sensitivity (%) | Specificity (%) | Accuracy (%) | Youden's index | |||||
|---|---|---|---|---|---|---|---|---|---|
| SC | AC | SCC | Lung cancer | SC | AC | SCC | |||
| ProGRP | 19.1 (13/68) | 19.4 (36/186) | 64.3 (45/70) | 30.9 (100/324) | 94.1 (48/51) | 51.3 (61/119) | 35.4 (84/237) | 76.9 (93/121) | 0.25 |
| NSE | 32.4 (22/68) | 28.0 (52/186) | 54.3 (38/70) | 35.2 (114/324) | 90.2 (46/51) | 57.1 (68/119) | 41.4 (98/237) | 69.4 (84/121) | 0.25 |
| CYFRA21-1 | 13.2 (9/68) | 40.3 (75/186) | 24.3 (17/70) | 40.7 (132/324) | 70.6 (36/51) | 37.8 (45/119) | 46.8 (111/237) | 43.8 (53/121) | 0.11 |
| CEA | 7.4 (5/68) | 60.2 (112/186) | 34.3 (24/70) | 46.9 (152/324) | 84.3 (43/51) | 40.3 (48/119) | 65.4 (155/237) | 55.4 (67/121) | 0.31 |
| SCCA | 8.8 (6/68) | 11.3 (21/186) | 8.6 (6/70) | 14.5 (47/324) | 86.3 (44/51) | 42.0 (50/119) | 27.4 (65/237) | 41.3 (50/121) | 0.01 |
| CA19-9 | 2.9 (2/68) | 28.5 (53/186) | 8.6 (6/70) | 19.2 (62/324) | 94.1 (48/51) | 42.0 (50/119) | 42.6 (101/237) | 44.6 (54/121) | 0.13 |
| ProGRP + NSE | 44.1 (30/68) | 40.3 (75/186) | 72.9 (51/70) | 50 (162/324) | 86.3 (44/51) | 62.2 (74/119) | 50.2 (119/237) | 78.5 (95/121) | 0.36 |
| ProGRP + NSE + CYFRA21-1 | 63.2 (43/68) | 54.8 (102/186) | 77.1 (54/70) | 63.9 (207/324) | 62.7 (32/51) | 63.0 (75/119) | 56.5 (134/237) | 71.1 (86/121) | 0.27 |
| ProGRP + NSE + CYFRA21-1 + CEA | 69.1 (47/68) | 74.7 (139/186) | 80.0 (56/70) | 75.9 (246/324) | 52.9 (27/51) | 62.2 (74/119) | 70.0 (166/237) | 68.6 (83/121) | 0.29 |
| ProGRP + NSE + CYFRA21-1 + CEA + SCCA | 70.6 (48/68) | 75.8 (141/186) | 80.0 (56/70) | 76.9 (249/324) | 47.1 (24/51) | 60.5 (72/119) | 69.6 (165/237) | 66.1 (80/121) | 0.24 |
| ProGRP + NSE + CYFRA21-1 + CEA + SCCA + CA19-9 | 70.6 (48/68) | 77.4 (144/186) | 80.0 (56/70) | 77.8 (252/324) | 45.1 (23/51) | 59.7 (71/119) | 70.5 (167/237) | 65.3 (79/121) | 0.23 |
- Abbreviations: AC, adenocarcinomas; CA19-9, cytokeratin 19 fragment; CEA, carcinoembryonic antigen; CYFRA21-1, cytokeratin 19 fragment; NSE, neuron-specific enolase; ProGRP, progastrin-releasing peptide; SC, squamous cell carcinoma; SCC, small cell carcinomas; SCCA, squamous cell carcinoma antigen.
The area under the ROC curve was computed to compare the diagnostic accuracies of ProGRP, NSE, CYFRA21-1, CEA, SCCA, and CA19-9 for the different types of pulmonary carcinoma. In SC, NSE exhibited a higher AUC value (0.719) than ProGRP (0.638), CYFRA21-1 (0.617), CEA (0.502), SCCA (0.584), or CA19-9 (0.446) (see Figure 1a). In the context of AC, CEA exhibited a higher AUC value (0.716) than ProGRP (0.619), CYFRA21-1 (0.579), NSE (0.701), SCCA (0.441), or CA19-9 (0.639) (see Figure 1b). Conversely, in SCC, ProGRP demonstrated a superior AUC value of 0.866 when compared with NSE (0.775), CYFRA21-1 (0.538), CEA (0.563), SCCA (0.474), and CA19-9 (0.461) (see Figure 1c).
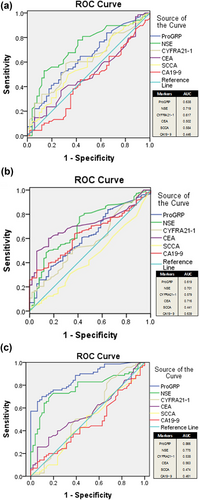
ROC curves of six tumor markers for pulmonary carcinoma. (a) SC, (b) AC, (c) SCC. ROC, receiver operator characteristic; SC, squamous cell carcinoma; SCC, small cell carcinomas.
4 DISCUSSION
In this study, the individual and combined performances of six common serum tumor markers (ProGRP, NSE, CA19-9, CEA, SCCA, and CYFRA21-1) were evaluated for lung cancer diagnosis. While sensitivity, specificity, and accuracy are commonly used measures, additional indices such as the ROC curve and Youden's index have been developed to offer more precise and comprehensive evaluations. ROC analysis is a powerful tool for evaluating the diagnostic accuracy of biomarkers. Our analyses showed that in patients with lung cancer but not those with benign lung disease, the serum levels of CYFRA21-1, SCCA, and CA19-9 were significantly higher than the levels of ProGRP, NSE, and CEA. Among these six tumor markers, NSE, CEA, and ProGRP demonstrated the highest levels of accuracy (57.1%, 65.4%, and 76.9%, respectively) and largest AUC values (0.719, 0.716, and 0.866, respectively) for the subtypes SC, AC, and SCC, respectively. However, the sensitivities of the six markers examined were all below 65%. Table 3 illustrates that combining tumor markers is a viable approach for enhancing sensitivity. The combination of ProGRP and NSE may be the optimal choice as it exhibited the highest Youden's index (0.36) with superior sensitivity (50.0%) and specificity (86.3%). In addition, the utilization of diverse marker combinations may aid in distinguishing between histological subtypes of pulmonary carcinoma. For example, to help the diagnosis of SC, the combination of ProGRP, NSE, CYFRA21-1, CEA, and SCCA was the most sensitive (70.6%) and exhibited better specificity (47.1%) than other combinations. Meanwhile, the combination of ProGRP, NSE, CYFRA21-1, CEA, SCCA, and CA19-9 (with a sensitivity of 77.4% and specificity of 45.1%) could be utilized as an adjunct in the diagnosis of AC. Finally, to aid in the diagnosis of SCC, the combination of ProGRP, NSE, CYFRA21-1, and CEA (with a sensitivity of 80.0% and specificity of 52.9%) seemed to be optimal.
The study demonstrated that among the six serum tumor markers, ProGRP exhibited the highest diagnostic sensitivity and specificity (64.3% and 94.1%) in SCC, followed by NSE (54.3% and 90.2%). One report [3] indicated that Pro-GRP levels were significantly higher in SCC patients than in non-small cell lung cancer (NSCLC) patients (p < 0.05). According to the latest findings, the diagnostic efficiencies of NSE and ProGRP for SCC are quite remarkable. The efficiency of NSE was found to be 0.8554, while ProGRP exhibited a slightly higher efficiency of 0.9053. Interestingly, when these two biomarkers were combined, their diagnostic efficiency increased to an impressive 0.9426. This combined efficiency surpassed the efficiency of NSE alone, indicating the potential of this combination as a more reliable diagnostic tool for SCC detection [4]. However, it is worth noting that despite this significant improvement in overall diagnostic performance with the combination approach, the disparity in efficiency between the combined markers and proGRP alone did not reach statistical significance. This result suggests that both biomarkers are of similar value in terms of accurately identifying SCC cases. These findings shed light on the importance of utilizing multiple biomarkers in the effective diagnosis of SCC. By leveraging the strengths and unique characteristics of each marker, healthcare professionals can enhance their ability to detect this aggressive form of lung cancer at an early stage. Further research is warranted to explore additional combinations/alternative markers that could potentially yield even higher diagnostic efficiencies and improve patient outcomes in the battle against SCC.
NSE can be used to distinguish between SCC and NSCLC. In a recent study, significant increases in NSE concentrations in lung cancer patients suggested that they were more likely to be suffering from SCC [5]. In line with the aforementioned conclusion, our study found significantly higher serum NSE levels in SCC patients than in SC or AC patients (p < 0.05).
CYFRA21-1 is a diagnostic marker that specifically detects fragments of cytokeratin 19, an essential protein found in the intermediate filament network of epithelial cells. Epithelial cells play a crucial role in maintaining the integrity and function of various organs and tissues within the body. In cases where these normally healthy epithelial cells transform into malignant or cancerous cells, there is an observed increase in keratin content. This phenomenon can be attributed to the abnormal growth and division of these transformed cells. Interestingly, when tumor cells experience necrosis (cell death), they release these soluble CYFRA21-1 fragments into the bloodstream. These fragments serve as valuable indicators that enable medical professionals to detect and monitor the presence of certain types of cancers. Nonetheless, it is important to note that while CYFRA21-1 provides useful information about cancerous conditions, it does not possess tissue-specific or tumor-specific characteristics. In other words, this biomarker cannot pinpoint the specific organ or type of tumor affected.
By understanding how CYFRA21-1 functions within our bodies, healthcare providers can utilize this knowledge to aid in diagnosing and monitoring patients with suspected or confirmed malignancies more effectively. The levels of CYFRA21-1 in serum tend to increase when epithelial cells transform into cancerous tumor cells. This phenomenon is particularly notable for squamous epithelial cells in the lungs and transitional cells in the bladder. However, our comprehensive study findings shed light on an intriguing aspect—the serum levels of CYFRA21-1 among individuals diagnosed with either lung cancer or benign lung disease did not exhibit any significant disparity (p > 0.05). These results suggest that CYFRA21-1 does not serve as a reliable biomarker for distinguishing between malignant and non-malignant conditions affecting the lungs, warranting further investigation into alternative diagnostic markers and techniques. Table 2 shows that the serum level of CYFRA21-1 was higher in SC than in SCC (p < 0.05).
Another study [6] has revealed compelling findings suggesting that CYFRA21-1 could serve as a valuable prognostic marker for individuals diagnosed with NSCLC who are undergoing chemotherapy treatment. This groundbreaking research shed light on the potential of utilizing CYFRA21-1 as an indicator to predict patient outcomes and guide personalized therapeutic approaches in NSCLC management. By incorporating this novel biomarker into clinical practice, healthcare professionals can enhance their ability to accurately assess prognosis and tailor treatment strategies accordingly, ultimately leading to improved patient care and better overall survival rates. Serum levels of CYFRA21-1 are commonly used in the diagnosis and monitoring of lung cancer and have been found to vary significantly across different pathological types. In particular, studies have shown that the levels of CYFRA21-1 are highest in SC, followed by AC and SCC. This variation in CYFRA21-1 levels can be attributed to differences in the expression patterns of cytokeratins CK-19 and CK-18. Specifically, SC and AC tumors tend to express high levels of CK-19, which is known to be a major component of intermediate filaments in epithelial cells, whereas SCC tumors typically express high levels of CK-18. Given these findings, it is clear that CYFRA21-1 has significant diagnostic value for identifying SC tumors specifically. However, it may also serve as a useful indicator for broader histological subtyping. Therefore, clinicians should consider measuring serum CYFRA21-1 levels when evaluating patients with suspected or confirmed lung cancer.
CEA, also known as CEA, is an acid glycoprotein that plays a crucial role in determining the specificity of human embryonic antigens. This protein belongs to a group of non-organ-specific tumor-associated antigens, which have been extensively studied for their potential implications in cancer diagnosis and treatment. CEA is primarily produced during fetal development but can also be found in small amounts in healthy adults. It serves as a marker for various types of cancers, including colorectal, pancreatic, lung, breast, and ovarian cancers. The presence of CEA in the bloodstream or other bodily fluids often indicates the presence or progression of these malignancies. The concentration of this particular substance in the bloodstream of a healthy adult is exceedingly low, while elevated levels are strongly correlated with the growth and spread of cancerous cells throughout the body [7]. In a study conducted by Pothal et al. [8], CEA levels in both bronchoalveolar lavage fluid and serum were found to serve as an effective screening tool for detecting malignancy. This discovery has significant implications for patients who may require further workup to determine whether they have bronchogenic carcinoma. Our data showed elevated levels (p < 0.05) and higher sensitivity (60.2%) for CEA in AC; the AUC for CEA in this study was 0.719.
SCCA, also known as squamous cell carcinoma antigen, has emerged as a prominent tumor marker. It has garnered significant attention because of its multifaceted biological functions and profound implications in both normal physiological processes and pathological conditions [9]. Xu et al. [10] revealed that the AUC of serum CEA was 0.825, indicating moderate accuracy in distinguishing between patients with and without lung AC; similarly, CYFRA21-1 showed an AUC value of 0.842, also suggesting good diagnostic potential for detecting lung AC. Interestingly, SCCA demonstrated the highest AUC value among all three biomarkers tested, with a score of 0.857, indicating excellent sensitivity and specificity for lung AC.
In our study, we did not observe a statistically significant difference in SCCA serum levels between the lung cancer group and the benign lung disease group. Moreover, considering the fact that SCCA exhibited the lowest sensitivity (8.8%, 11.3%, and 8.6% in SC, AC, and SCC, respectively) as well as the lowest Youden's index (0.01), it may not be a reliable marker for lung cancer screening. However, the serum level of SCCA was found to be significantly higher in SC than in SCC and AC (p < 0.05), so it could potentially serve as a valuable marker for histological subtyping. Nonetheless, because of its significantly reduced sensitivity, it would be preferable to use SCCA in conjunction with CYFRA21-1, which is also specific for SC.
CA19-9 has been used as a tumor marker in the diagnosis of pancreatic cancers, and increased levels of CA19-9 have been described in other malignancies. In a groundbreaking study conducted by Zhou and his team [11], preoperative serum CA19-9 levels were discovered to serve as a crucial indicator of prognostic outcome for patients suffering from stage III colon cancer. The use of CA19-9 as a serum biomarker has been approved by the US Food and Drug Administration. However, it is important to note that this marker should not be used as an early screening tool for pancreatic cancer because of its limited specificity [12]. Our data showed low specificity and sensitivity for CA19-9 in lung cancer, but elevated levels in AC (p < 0.05) were confirmed.
Although combining tumor markers can enhance sensitivity, it may compromise specificity and escalate costs. Using the optimal combination of markers is not only beneficial for enhancing the diagnostic efficiency but also for alleviating the economic burden on patients and healthcare management departments. Some reports [13, 14] suggest that cost-effectiveness analyses should be conducted to evaluate combinations of tumor markers. Our data suggest that the combination of ProGRP, NSE, CYFRA21-1, CEA, and SCCA is the optimal choice for SC, given its high sensitivity (70.6%) and higher Youden's index (0.24). For diagnosing SCC, the Youden's index and specificity of the six-marker panel were inferior to those of the four-marker panel (ProGRP + NSE + CEA + CYFRA21-1). For AC, the combination of ProGRP, NSE, CYFRA21-1, CEA, SCCA, and CA19-9 was superior to any other panel because of the diagnostic value of CA19-9.
Lung cancer has a dismal prognosis and represents a significant public health challenge in developing nations. Many innovative techniques have been extensively utilized in the detection of lung cancer, aiming to provide accurate and timely diagnoses. These include widely recognized methods such as chest radiography (X-ray), computed tomography, magnetic resonance imaging, sputum cytology, and bronchoscopy. However, it is important to acknowledge that these approaches often pose certain limitations because of their invasive nature, high costs, and time-consuming procedures. To overcome these challenges and enhance patient comfort during screening processes, non-invasive blood tests have emerged as a promising alternative in clinical settings. By analyzing specific biomarkers present in the bloodstream, medical professionals can effectively screen for lung cancer without resorting to invasive measures that may potentially damage delicate bronchial structures or the lungs themselves. The use of non-invasive blood tests not only offers convenience but also provides an opportunity for the early detection of lung cancer. This is crucial as early diagnosis significantly improves treatment outcomes by enabling prompt intervention when the disease is still at its initial stages. Moreover, these blood-based screenings offer a more comprehensive assessment by examining various molecular markers associated with lung cancer development. Incorporating non-invasive blood tests into routine screening protocols also facilitates the regular monitoring of individuals at higher risk or with a family history of lung cancer. Nonetheless, although the detection of tumor markers in blood is a quick and easy method of diagnosis, no single tumor marker has sufficient diagnostic accuracy. In addition, most tumor markers appear to be associated with tumors rather than specific to them. Joint detection of tumor markers has been widely utilized in clinical practice for the early diagnosis and pathological typing of lung cancer.
In recent years, there has been a surge of interest in identifying new serum biomarkers for the early detection of lung cancer. Researchers have conducted numerous studies to uncover novel combinations that can improve accuracy and reliability. In addition to the identification of new markers, liquid biopsy techniques are gaining traction as a promising avenue for detecting lung cancer. These methods involve analyzing circulating tumor cells, circulating tumor DNA, microRNAs, and exosomes found in blood samples. The potential benefits of liquid biopsies are manifold: they are minimally invasive, offer real-time monitoring, and can provide valuable insights into disease progression and treatment response. In addition to these cutting-edge approaches, researchers are exploring other innovative technologies such as artificial intelligence, computer-aided diagnosis systems, metabolomics technology, and ion mobilization. By combining these tools with traditional diagnostic methods, we may be able to develop more accurate and effective ways of detecting lung cancer at an earlier stage, ultimately improving patient outcomes. Therefore, conducting a large-scale validation study on other novel tumor markers would be a valuable avenue for future research.
AUTHOR CONTRIBUTIONS
Yongchang Yang and Shuai Chang analyzed the data and prepared the manuscript. Jie Liu performed the experimental designs, Na Wang and Pengfei Song performed many of the experiments, and Haijing Wei participated in data collection and organization. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We would like to thank all those who participated in the study for their time and involvement.
CONFLICT OF INTEREST STATEMENT
Professor Jie Liu is a member of the iLABMED Editorial Board. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining authors declare no conflicts of interest.
ETHICS STATEMENT
The studies involving human participants were reviewed and approved by the Seventh Medical Center of Chinese PLA General Hospital, and the approved number was 2016-62.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used or analyzed in this study are available from the corresponding author upon reasonable request.




