Targeting erythroid progenitor cells for cancer immunotherapy
Su-Ran Li
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, Hubei, P. R. China
Contribution: Conceptualization, Visualization, Writing - original draft, Writing - review & editing
Search for more papers by this authorZhi-Zhong Wu
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, Hubei, P. R. China
Contribution: Writing - original draft, Writing - review & editing
Search for more papers by this authorCorresponding Author
Hai-Jun Yu
Department of Radiation and Medical Oncology, Hubei Provincial Clinical Research Center for Cancer, Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, P. R. China
Correspondence
Hai-Jun Yu, Department of Radiation and Medical Oncology, Hubei Provincial Clinical Research Center for Cancer, Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China.
Email: [email protected]
Zhi-Jun Sun, State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, 430079, Hubei, P. R. China.
Email: [email protected]
Contribution: Conceptualization, Resources, Writing - review & editing
Search for more papers by this authorCorresponding Author
Zhi-Jun Sun
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, Hubei, P. R. China
Correspondence
Hai-Jun Yu, Department of Radiation and Medical Oncology, Hubei Provincial Clinical Research Center for Cancer, Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China.
Email: [email protected]
Zhi-Jun Sun, State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, 430079, Hubei, P. R. China.
Email: [email protected]
Contribution: Conceptualization, Resources, Visualization, Writing - review & editing
Search for more papers by this authorSu-Ran Li
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, Hubei, P. R. China
Contribution: Conceptualization, Visualization, Writing - original draft, Writing - review & editing
Search for more papers by this authorZhi-Zhong Wu
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, Hubei, P. R. China
Contribution: Writing - original draft, Writing - review & editing
Search for more papers by this authorCorresponding Author
Hai-Jun Yu
Department of Radiation and Medical Oncology, Hubei Provincial Clinical Research Center for Cancer, Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, P. R. China
Correspondence
Hai-Jun Yu, Department of Radiation and Medical Oncology, Hubei Provincial Clinical Research Center for Cancer, Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China.
Email: [email protected]
Zhi-Jun Sun, State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, 430079, Hubei, P. R. China.
Email: [email protected]
Contribution: Conceptualization, Resources, Writing - review & editing
Search for more papers by this authorCorresponding Author
Zhi-Jun Sun
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, Hubei, P. R. China
Correspondence
Hai-Jun Yu, Department of Radiation and Medical Oncology, Hubei Provincial Clinical Research Center for Cancer, Hubei Key Laboratory of Tumor Biological Behaviors, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China.
Email: [email protected]
Zhi-Jun Sun, State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Frontier Science Center for Immunology and Metabolism, Taikang Center for Life and Medical Sciences, Wuhan University, Wuhan, 430079, Hubei, P. R. China.
Email: [email protected]
Contribution: Conceptualization, Resources, Visualization, Writing - review & editing
Search for more papers by this authorSu-Ran Li and Zhi-Zhong Wu authors contributed equally to this work.
Abstract
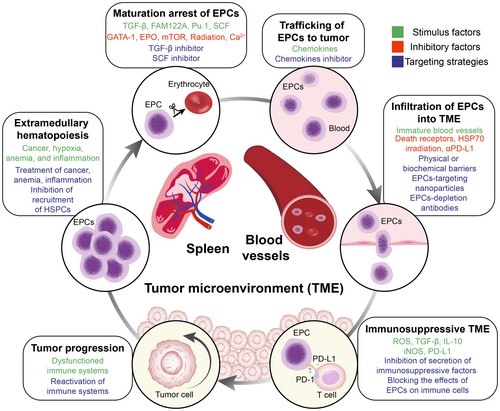
Immunotherapy, especially immune checkpoint blockade therapy, represents a major milestone in the history of cancer therapy. However, the current response rate to immunotherapy among cancer patients must be improved; thus, new strategies for sensitizing patients to immunotherapy are urgently needed. Erythroid progenitor cells (EPCs), a population of immature erythroid cells, exert potent immunosuppressive functions. As a newly recognized immunosuppressive population, EPCs have not yet been effectively targeted. In this review, we summarize the immunoregulatory mechanisms of EPCs, especially for CD45+ EPCs. Moreover, in view of the regulatory effects of EPCs on the tumor microenvironment, we propose the concept of EPC-immunity, present existing strategies for targeting EPCs, and discuss the challenges encountered in both basic research and clinical applications. In particular, the impact of existing cancer treatments on EPCs is discussed, laying the foundation for combination therapies. The aim of this review is to provide new avenues for improving the efficacy of cancer immunotherapy by targeting EPCs.
Graphical Abstract
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1Rodriguez-Garcia A, Lynn RC, Poussin M, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021; 12: 877.
- 2Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009; 58: 49-59.
- 3Tesi RJ. MDSC; the Most important cell you have never heard of. Trends Pharmacol Sci. 2019; 40: 4-7.
- 4Krishnamoorthy M, Gerhardt L, Maleki Vareki S. Immunosuppressive effects of myeloid-derived suppressor cells in cancer and immunotherapy. Cells. 2021; 10:1170.
- 5Li K, Shi H, Zhang B, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021; 6: 362.
- 6Liu C, Chikina M, Deshpande R, et al. Treg cells promote the SREBP1-dependent metabolic fitness of tumor-promoting macrophages via repression of CD8+ T cell-derived interferon-γ. Immunity. 2019; 51: 381-397.e6.
- 7Wang H, Franco F, Tsui Y-C, et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat Immunol. 2020; 21: 298-308.
- 8Sano Y, Yoshida T, Choo M-K, et al. Multiorgan signaling mobilizes tumor-associated erythroid cells expressing immune checkpoint molecules. Mol Cancer Res. 2021; 19: 507-515.
- 9Zhao L, He R, Long H, et al. Late-stage tumors induce anemia and immunosuppressive extramedullary erythroid progenitor cells. Nat Med. 2018; 24: 1536-1544.
- 10Han Y, Liu Q, Hou J, et al. Tumor-induced generation of splenic erythroblast-like Ter-cells promotes tumor progression. Cell. 2018; 173: 634-648.e12.
- 11Chen J, Qiao Y-D, Li X, et al. Intratumoral CD45+CD71+ erythroid cells induce immune tolerance and predict tumor recurrence in hepatocellular carcinoma. Cancer Lett. 2021; 499: 85-98.
- 12Bozorgmehr N, Okoye I, Mashhouri S, et al. CD71(+) erythroid cells suppress T-cell effector functions and predict immunotherapy outcomes in patients with virus-associated solid tumors. J Immunother Cancer. 2023; 11:e006595.
- 13Yang X, Chen D, Long H, Zhu B. The mechanisms of pathological extramedullary hematopoiesis in diseases. Cell Mol Life Sci. 2020; 77: 2723-2738.
- 14Gallagher PG. Extramedullary hematopoietic stem cells. Blood. 2022; 139: 3353-3354.
- 15Yamashita M, Dellorusso P, Olson O, Passegué E. Dysregulated haematopoietic stem cell behaviour in myeloid leukaemogenesis. Nat Rev Cancer. 2020; 20: 365-382.
- 16Hiam-Galvez K, Allen B, Spitzer M. Systemic immunity in cancer. Nat Rev Cancer. 2021; 21: 345-359.
- 17Grzywa TM, Sosnowska A, Rydzynska Z, et al. Potent but transient immunosuppression of T-cells is a general feature of CD71+ erythroid cells. Commun Biol. 2021; 4: 1384.
- 18Bao Y, Liu Z, Guo M, Li B, Sun X, Wang L. Extramedullary hematopoiesis secondary to malignant solid tumors: a case report and literature review. Cancer Manag Res. 2018; 10: 1461-1470.
- 19Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013; 504: 158-162.
- 20Seledtsov VI, Seledtsova GV, Samarin DM, et al. Erythroid cells in suppressing leukemia cell growth. Leuk Lymphoma. 2005; 46: 1353-1356.
- 21Grzywa TM, Justyniarska M, Nowis D, Golab J. Tumor immune evasion induced by dysregulation of erythroid progenitor cells development. Cancers (Basel). 2021; 13:870.
- 22Long H, Jia Q, Wang L, et al. Tumor-induced erythroid precursor-differentiated myeloid cells mediate immunosuppression and curtail anti-PD-1/PD-L1 treatment efficacy. Cancer Cell. 2022; 40: 674-693.e7.
- 23Mello F, Land M, Costa E, et al. Maturation-associated gene expression profiles during normal human bone marrow erythropoiesis. Cell Death Discov. 2019; 5: 69.
- 24Saito S, Shahbaz S, Osman M, et al. Diverse immunological dysregulation, chronic inflammation, and impaired erythropoiesis in long COVID patients with chronic fatigue syndrome. J Autoimmun. 2024; 147:103267.
- 25Wang Q, Poole RA, Opyrchal M. Understanding and targeting erythroid progenitor cells for effective cancer therapy. Curr Opin Hematol. 2023; 30: 137-143.
- 26Elahi S. Neglected cells: immunomodulatory roles of CD71+ erythroid cells. Trends Immunol. 2019; 40: 181-185.
- 27Conway de Macario E, Macario AJL. A new kind of immunosuppression associated with erythropoiesis. Immunol Lett. 1979; 1: 23-26.
10.1016/0165-2478(79)90032-4 Google Scholar
- 28Delyea C, Bozorgmehr N, Koleva P, et al. CD71+ erythroid suppressor cells promote Fetomaternal tolerance through arginase-2 and PDL-1. J Immunol. 2018; 200: 4044-4058.
- 29Dunsmore G, Koleva P, Ghobakhloo N, et al. Lower abundance and impaired function of CD71+ erythroid cells in inflammatory bowel disease patients during pregnancy. J Crohns Colitis. 2019; 13: 230-244.
- 30Shahbaz S, Bozorgmehr N, Koleva P, et al. CD71+VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF-β. PLoS Biol. 2018; 16:e2006649.
- 31Cheng X, Wang H, Wang Z, Zhu B, Long H. Tumor-associated myeloid cells in cancer immunotherapy. J Hematol Oncol. 2023; 16: 71.
- 32Elahi S, Vega-López MA, Herman-Miguel V, et al. CD71+ erythroid cells in human neonates exhibit immunosuppressive properties and compromise immune response against systemic infection in neonatal mice. Front Immunol. 2020; 11:597433.
- 33Dunsmore G, Bozorgmehr N, Delyea C, Koleva P, Namdar A, Elahi S. Erythroid suppressor cells compromise neonatal immune response against Bordetella pertussis. J Immunol. 2017; 199: 2081-2095.
- 34Namdar A, Koleva P, Shahbaz S, Strom S, Gerdts V, Elahi S. CD71+ erythroid suppressor cells impair adaptive immunity against Bordetella pertussis. Sci Rep. 2017; 7: 7728.
- 35Mashhouri S, Koleva P, Huynh M, Okoye I, Shahbaz S, Elahi S. Sex matters: physiological abundance of immuno-regulatory CD71+ erythroid cells impair immunity in females. Front Immunol. 2021; 12:705197.
- 36Elahi S, Mashhouri S. Immunological consequences of extramedullary erythropoiesis: immunoregulatory functions of CD71+ erythroid cells. Haematologica. 2020; 105: 1478-1483.
- 37Shahbaz S, Xu L, Osman M, et al. Erythroid precursors and progenitors suppress adaptive immunity and get invaded by SARS-CoV-2. Stem Cell Rep. 2021; 16: 1165-1181.
- 38Saito S, Shahbaz S, Sligl W, Osman M, Tyrrell DL, Elahi S. Differential impact of SARS-CoV-2 isolates, namely, the Wuhan strain, Delta, and omicron variants on erythropoiesis. Microbiol Spectr. 2022; 10:e0173022.
- 39Wang Y, Xiang X, Chen H, et al. Intratumoral erythroblastic islands restrain anti-tumor immunity in hepatoblastoma. Cell Rep Med. 2023; 4:101044.
- 40Grzywa TM, Nowis D, Golab J. The role of CD71(+) erythroid cells in the regulation of the immune response. Pharmacol Ther. 2021; 228:107927.
- 41Yuan T, Jia Q, Zhu B, Chen D, Long H. Synergistic immunotherapy targeting cancer-associated anemia: prospects of a combination strategy. Cell Commun Signal. 2023; 21: 117.
- 42Li Y, Yue H, Yang S, et al. Splenomegaly induced by anemia impairs T cell movement in the spleen partially via EPO. Mol Immunol. 2019; 112: 399-405.
- 43Hare GMT, Mazer CD. Anemia: perioperative risk and treatment opportunity. Anesthesiology. 2021; 135: 520-530.
- 44Ferrario E, Ferrari L, Bidoli P, et al. Treatment of cancer-related anemia with epoetin alfa: a review. Cancer Treat Rev. 2004; 30: 563-575.
- 45Swann JW, Koneva LA, Regan-Komito D, Sansom SN, Powrie F, Griseri T. IL-33 promotes anemia during chronic inflammation by inhibiting differentiation of erythroid progenitors. J Exp Med. 2020; 217:e20200164.
- 46Tsiftsoglou AS. Erythropoietin (EPO) as a key regulator of erythropoiesis, bone remodeling and endothelial transdifferentiation of multipotent mesenchymal stem cells (MSCs): implications in regenerative medicine. Cells. 2021; 10:2140.
- 47Yan H, Ali A, Blanc L, et al. Comprehensive phenotyping of erythropoiesis in human bone marrow: evaluation of normal and ineffective erythropoiesis. Am J Hematol. 2021; 96: 1064-1076.
- 48Kuhikar R, Khan N, Philip J, Melinkeri S, Kale V, Limaye L. Transforming growth factor β1 accelerates and enhances in vitro red blood cell formation from hematopoietic stem cells by stimulating mitophagy. Stem Cell Res Ther. 2020; 11: 71.
- 49Xu Y, Wang B, Zhang M, et al. Carbon dots as a potential therapeutic agent for the treatment of cancer-related anemia. Adv Mater. 2022; 34:e2200905.
- 50Lam LKM, Murphy S, Kokkinaki D, et al. DNA binding to TLR9 expressed by red blood cells promotes innate immune activation and anemia. Sci Transl Med. 2021; 13:eabj1008.
- 51Zhang H, Wan G-Z, Wang Y-Y, Chen W, Guan J-Z. The role of erythrocytes and erythroid progenitor cells in tumors. Open Life Sci. 2022; 17: 1641-1656.
- 52Zhang J, Supakorndej T, Krambs JR, et al. Bone marrow dendritic cells regulate hematopoietic stem/progenitor cell trafficking. J Clin Invest. 2019; 129: 2920-2931.
- 53Ito S, Sato T, Maeta T. Role and therapeutic targeting of SDF-1α/CXCR4 Axis in multiple myeloma. Cancers (Basel). 2021; 13: 1793.
- 54Hidalgo A, Sanz-Rodríguez F, Rodríguez-Fernández JL, et al. Chemokine stromal cell-derived factor-1alpha modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001; 29: 345-355.
- 55Mende N, Bastos HP, Santoro A, et al. Unique molecular and functional features of extramedullary hematopoietic stem and progenitor cell reservoirs in humans. Blood. 2022; 139: 3387-3401.
- 56Wu C, Ning H, Liu M, et al. Spleen mediates a distinct hematopoietic progenitor response supporting tumor-promoting myelopoiesis. J Clin Invest. 2018; 128: 3425-3438.
- 57Wang B, Wang Y, Chen H, et al. Inhibition of TGFβ improves hematopoietic stem cell niche and ameliorates cancer-related anemia. Stem Cell Res Ther. 2021; 12: 65.
- 58Gutiérrez L, Caballero N, Fernández-Calleja L, Karkoulia E, Strouboulis J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life. 2020; 72: 89-105.
- 59Chen J, Zhou Q, Liu MH, et al. FAM122A inhibits erythroid differentiation through GATA1. Stem Cell Rep. 2020; 15: 721-734.
- 60He S, Wang T, Shi C, Wang Z, Fu X. Network pharmacology-based approach to understand the effect and mechanism of Danshen against anemia. J Ethnopharmacol. 2022; 282:114615.
- 61Tóthová Z, Tomc J, Debeljak N, Solár P. STAT5 as a key protein of erythropoietin signalization. Int J Mol Sci. 2021; 22:7109.
- 62Willcockson MA, Taylor SJ, Ghosh S, et al. Runx1 promotes murine erythroid progenitor proliferation and inhibits differentiation by preventing Pu.1 downregulation. Proc Natl Acad Sci U S A. 2019; 116: 17841-17847.
- 63Dulmovits BM, Tang Y, Papoin J, et al. HMGB1-mediated restriction of EPO signaling contributes to anemia of inflammation. Blood. 2022; 139: 3181-3193.
- 64Comazzetto S, Murphy MM, Berto S, Jeffery E, Zhao Z, Morrison SJ. Restricted hematopoietic progenitors and erythropoiesis require SCF from leptin receptor+ niche cells in the bone marrow. Cell Stem Cell. 2019; 24: 477-486.e6.
- 65Zhang Y, Xu Y, Zhang S, Lu Z, Li Y, Zhao B. The regulation roles of Ca2+ in erythropoiesis: what have we learned? Exp Hematol. 2022; 106: 19-30.
- 66Feng Y, Borosha S, Ratri A, et al. DOT1L Methyltransferase regulates calcium influx in erythroid progenitor cells in response to erythropoietin. Int J Mol Sci. 2022; 23:5137.
- 67Singbrant S, Mattebo A, Sigvardsson M, Strid T, Flygare J. Prospective isolation of radiation induced erythroid stress progenitors reveals unique transcriptomic and epigenetic signatures enabling increased erythroid output. Haematologica. 2020; 105: 2561-2571.
- 68Cho WC, Mandavilli S. Intratumoral extramedullary hematopoiesis in solitary fibrous tumor of the breast. Breast J. 2020; 26: 755-758.
- 69Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012; 49: 508-523.
- 70Cruz LJ, van Dijk T, Vepris O, et al. PLGA-nanoparticles for intracellular delivery of the CRISPR-complex to elevate fetal globin expression in erythroid cells. Biomaterials. 2021; 268:120580.
- 71Chen Q, Sun T, Jiang C. Recent advancements in Nanomedicine for ‘Cold’ tumor immunotherapy. Nanomicro Lett. 2021; 13: 92.
- 72Mathangasinghe Y, Fauvet B, Jane SM, Goloubinoff P, Nillegoda NB. The Hsp70 chaperone system: distinct roles in erythrocyte formation and maintenance. Haematologica. 2021; 106: 1519-1534.
- 73Hou Y, Liang HL, Yu X, et al. Radiotherapy and immunotherapy converge on elimination of tumor-promoting erythroid progenitor cells through adaptive immunity. Sci Transl Med. 2021; 13:eabb0130.
- 74Sofias AM, Toner YC, Meerwaldt AE, et al. Tumor targeting by αvβ3-integrin-specific lipid nanoparticles occurs via phagocyte hitchhiking. ACS Nano. 2020; 14: 7832-7846.
- 75Yang L, Shivakumar P, Kinder J, et al. Regulation of bile duct epithelial injury by hepatic CD71+ erythroid cells. JCI Insight. 2020; 5:e135751.
- 76Perera SK, Jacob S, Wilson BE, et al. Global demand for cancer surgery and an estimate of the optimal surgical and anaesthesia workforce between 2018 and 2040: a population-based modelling study. Lancet Oncol. 2021; 22: 182-189.
- 77Barisas DAG, Kabir AU, Wu J, et al. Tumor-derived interleukin-1α and leukemia inhibitory factor promote extramedullary hematopoiesis. PLoS Biol. 2023; 21:e3001746.
- 78Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies. Trends Cancer. 2022; 8: 9-20.
- 79Nimker S, Sharma K, Saraswathy R, Chandna S. Delineating the effects of ionizing radiation on Erythropoietic lineage-implications for radiation biodosimetry. Health Phys. 2019; 116: 677-693.
- 80Lövey J, Bereczky B, Gilly R, et al. Recombinant human erythropoietin alpha improves the efficacy of radiotherapy of a human tumor xenograft, affecting tumor cells and microvessels. Strahlenther Onkol. 2008; 184: 1-7.
- 81Shen S-J, Liu C-M. Chemotherapy for early-stage breast cancer: the more the better? Lancet. 2023; 401: 1243-1245.
- 82Littlewood TJ. Management options for cancer therapy-related anaemia. Drug Saf. 2002; 25: 525-535.
- 83Abdel-Razeq H, Hashem H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol. 2020; 145:102837.
- 84Xie Y, Gao L, Xu C, et al. ARHGEF12 regulates erythropoiesis and is involved in erythroid regeneration after chemotherapy in acute lymphoblastic leukemia patients. Haematologica. 2020; 105: 925-936.
- 85Doleschel D, Rix A, Arns S, et al. Erythropoietin improves the accumulation and therapeutic effects of carboplatin by enhancing tumor vascularization and perfusion. Theranostics. 2015; 5: 905-918.
- 86Kanemasa H, Ishimura M, Eguchi K, et al. The immunoregulatory function of peripheral blood CD71+ erythroid cells in systemic-onset juvenile idiopathic arthritis. Sci Rep. 2021; 11:14396.