Explainable machine learning model for pre-frailty risk assessment in community-dwelling older adults
Chenlin Du, Zeyu Zhang, and Baoqin Liu contributed equally to this study.
Abstract
Background
Frailty in older adults is linked to increased risks and lower quality of life. Pre-frailty, a condition preceding frailty, is intervenable, but its determinants and assessment are challenging. This study aims to develop and validate an explainable machine learning model for pre-frailty risk assessment among community-dwelling older adults.
Methods
The study included 3141 adults aged 60 or above from the China Health and Retirement Longitudinal Study. Pre-frailty was characterized by one or two criteria from the physical frailty phenotype scale. We extracted 80 distinct features across seven dimensions to evaluate pre-frailty risk. A model was constructed using recursive feature elimination and a stacking-CatBoost distillation module on 80% of the sample and validated on a separate 20% holdout data set.
Results
The study used data from 2508 community-dwelling older adults (mean age, 67.24 years [range, 60–96]; 1215 [48.44%] females) to develop a pre-frailty risk assessment model. We selected 57 predictive features and built a distilled CatBoost model, which achieved the highest discrimination (AUROC: 0.7560 [95% CI: 0.7169, 0.7928]) on the 20% holdout data set. The living city, BMI, and peak expiratory flow (PEF) were the three most significant contributors to pre-frailty risk. Physical and environmental factors were the top 2 impactful feature dimensions.
Conclusions
An accurate and interpretable pre-frailty risk assessment framework using state-of-the-art machine learning techniques and explanation methods has been developed. Our framework incorporates a wide range of features and determinants, allowing for a comprehensive and nuanced understanding of pre-frailty risk.
Abbreviations
-
- ANN
-
- artificial neural network
-
- AUROC
-
- area under the receiver operator characteristic curve
-
- AutoML
-
- auto machine learning
-
- CatBoost
-
- Categorical Boosting
-
- CHARLS
-
- China Health and Retirement Longitudinal Study
-
- CI
-
- confidence interval
-
- KD
-
- knowledge distillation
-
- LightGBM
-
- Light Gradient-Boosting Machine
-
- LR
-
- logistics regression
-
- ML
-
- machine learning
-
- PACIFIC
-
- interPretable ACcurate and effICient pre-FraIlty Classification
-
- PEF
-
- peak expiratory flow
-
- PFP
-
- physical frailty phenotype
-
- RF
-
- Random Forest
-
- RFE
-
- recursive feature elimination
-
- SAGE
-
- shapley additive global explanation
-
- SHAP
-
- SHapley Additive exPlanations
-
- XAI
-
- explainable artificial intelligence
-
- XGBoost
-
- eXtreme Gradient Bboosting
-
- XT
-
- eXtreme randomized tree
1 BACKGROUND
Frailty is a complex age-related clinical condition characterized by a decline in physiological capacity across several organ systems, with a resultant increased susceptibility to stressors [1]. Older adults with frailty have an increased likelihood of unmet care needs, falls and fractures, hospitalizations, lower quality of life, and early mortality [2, 3]. Pre-frailty is an evident risk-state before the onset of clinically identifiable frailty [4]. In China, the rapid expansion of the aging population has led to an increasing prevalence of frailty and pre-frailty, with the prevalence of 10% and 43%, respectively [5]. Evidence suggests that comparing with frail individuals, the pre-frail older adults are more susceptible to intervention, which can prevent and delay frailty or even reverse the state from pre-frailty to robust [6]. Therefore, effective strategies that target early detection and prevention of pre-frailty in an aging population become China's national priority to reduce the condition burden at the level of both individual and health system.
The emergence of machine learning (ML) in the field of pre-frailty is a relatively recent development [7, 8]. It has long been recognized that pre-frailty in the clinical population is heterogeneous in terms of physical, psychosocial, demographic, and environmental characteristics. The profiling focuses on understanding the individual's unique combination of characteristics and their association with pre-frailty. Traditional pre-frailty assessment scales cannot determine or predict pre-frailty risk with an acceptable degree of precision or reliability. The development of ML in pre-frailty research represents an important methodological advance in the field of precision health care as it improves the accuracy and potential clinical utility [9-12]. Given the additional pressure of the COVID-19 pandemic on already overstretched healthcare services, potential gains in scalable quality of care and improvements to resource efficiency are appealing.
Despite their proven effect, real-world implementation of ML into frailty research still faces three barriers. First, although the research agenda of the determinants of frailty keeps expanding, most of the studies focus on the identification of frailty instead of more intervenable pre-frail individuals. Second, we know little about the relative importance of individual characteristics, built environment, social-economic contexts, and social policies that have only been examined in a piecemeal fashion. It is important to understand which factors exert the most important influence on pre-frailty among a large number of candidate predictors. Third, due to a lack of algorithm transparency, limited interpretability, and theoretical frameworks, clinicians are reluctant to trust the tools being integrated into care settings. The clinical implications of ML and how these techniques might be applied for early detection and intervention have largely been overlooked.
In this study, we drew data from the China Health and Retirement Longitudinal Study (CHARLS) to identify the most important determinants of pre-frailty for Chinese older adults from a large set of factors at biological, individual, and community levels. We took advantage of the recursive feature selection method to estimate the importance of factors cutting across multiple dimensions. We focus on the practical aspects of how explainable artificial intelligence can be clinically useful to increase model interpretability.
2 METHODS
2.1 Data source
The data were collected from the baseline survey (2011–2012) of CHARLS [13], an ongoing longitudinal cohort study of nationally representative community-dwelling adults aged 45 or older from China with comprehensive biomedical, clinical, and sociodemographic information. All participants were provided with written consent and the study protocol was approved by the Ethical Review Committee at Peking University (IRB00001052−11015).
It had been previously reported that the missing data can impact the interpretation of the prediction model [14]. Therefore, to avoid the potential bias, the current study only included participants without missing information. The overall exclusion criteria were as follows: (1) younger than 60 years old, (2) did not complete 2 or more components in physical frailty phenotype (PFP) scale [15], (3) categorized as frailty by PFP scale, and (4) missing information present among selected features. We randomly selected 20% of the included subjects as the test set while the rest of them (80%) as the training set.
2.2 Frailty level measurements
The frailty level of each participant was measured by the PFP scale [16] (validated on CHARLS [15]), in which five elements included weakness, slowness, exhaustion, inactivity, and shrinking (Supporting Information S1: Tables S1 and S2). Individuals with no criteria met were categorized as robust; those with one or two were classified as prefrail and three to five were considered frail. Details of frailty measures and cutoffs are shown in the supplementary methods.
2.3 Determinants of pre-frailty
Through extensive literature review and expert consultation (Z.Z. and J.N.), we summarized the following 80 features from seven dimensions [1] including laboratory measurements, physical factors, demographics, health behaviors, comorbidities and medical histories, healthcare service utilization and environmental factors for the analysis. 80 features are detailed in the supplementary methods.
2.4 Analytical plan
As demonstrated in Figure 1, we optimize the IMPACT framework [19] by introducing Categorical Boosting (CatBoost) [20] to present the interPretable ACcurate and effICient pre-FraIlty Classification (PACIFIC) framework, which enables analysis of categorical features. Moreover, with the help of knowledge distillation [21] (KD) we introduced a stacking-CatBoost distillation module (Figure 1b) to improve the model performance. In the module, we first train several different tree-based models including Random Forest (RF), eXtreme Gradient Boosting (XGBoost), Light Gradient-Boosting Machine (LightGBM), CatBoost, eXtreme randomized Tree (XT) with k-fold bagging [22] as base model and stacked them using ensemble selection [23]. Whereafter, the stacking model is distilled into a CatBoost model to enable efficient calculation, analyses of categorical features and feature interaction effects. Hyperparameters for different models were illustrated in Supporting Information S1: Table S3.
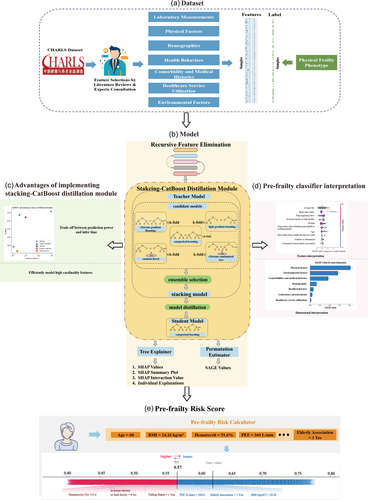
2.5 Recursive feature elimination (RFE)
Several literatures [19, 24] have demonstrated the efficiency of task-aware supervised features selection. In the current study, to ensure the time efficiency, a LighGBM [25] with fast training and high accuracy is applied to recursively eliminate features. In detail, we generate a random feature which follows the standard normal distribution first and then train the model, obtaining the area under the receiver operator characteristic curve (AUROC). After then, we rank the feature importance with the help of permutation feature importance [26]. We then remove up to 5% of the total features whose feature importance is below the randomly generated feature from the bottom of the feature importance ranked list. A new model is then trained with the remaining features with AUROC evaluated. If the AUROC of the new model is not improved, we then randomly choose up to 5% features, with the sampling rate inversely proportional to their feature importance (feature with lowest feature importance has the highest probability being sampled). Once the AUROC of the model improved after removing the selected feature, the feature importance is ranked again using permutation feature importance with selected features removed. We end the RFE if the early stopping criteria have been met, or all the features set below the randomly generated feature have been evaluated without improving the model performance. We keep the k-fold bagging the same as the stacking model to ensure the data consistency [27].
2.6 Classifier modeling
For the purposes of accurate classification and analysis of categorical feature [28], a CatBoost [20] distilled from a stacking model is implemented to model the pre-frailty risk. AutoGluon-Tabular (v 0.7.0), an auto machine learning (AutoML) framework, is utilized to ensure the accurate and efficient training of the stacking model from different tree-based individual model via stacking, bagging, boosting and weighted combination. Whereafter, we distill our stacking model (teacher model) into a CatBoost (student model) with soft targets [21]. To demonstrate the accuracy of the distilled CatBoost, we also train RF, XGBoost, LightGBM, origin CatBoost (trained directly without knowledge distillation), XT, artificial neural network (ANN), and logistics regression (LR) for comparison. The hyperparameters (Supporting Information S1: Table S3) for each model are chosen by Random Search with 100 iterations and fivefold cross-validation. Model performance is measured with the AUROC, with accuracy, F1 score, precision and recall reported. The results are reported with mean values and a 95% confidence interval (CI) obtained from 1000 bootstrap samples for the test set. All individual models are build using the Scikit-learn package in Python 3.10. Simultaneously, we train the stacking and distilled CatBoost using AutoGluon-Tabular package. Note that, we keep the hyperparameters of distilled CatBoost the same as the directly trained CatBoost to evaluate the performance of KD. We also compare the infer time of stacking model, distilled CatBoost, XGBoost, ANN and LR to demonstrate the trade-off between model performance and computational efficiency.
2.7 Performance evaluation
To address the robust of the RFE methods, accuracy, AUROC, F1 score, precision and recall rate of the stacking model on the test set before and after the feature selection was reported. We also compare the accuracy, AUROC, F1 score, precision, and recall rate of stacking model, distilled CatBoost (trained with stacking-CatBoost distillation module), RF, XGBoost, LightGBM, origin CatBoost (trained directly without model distillation), ANN, XT and LR. The hyperparameters of the above models were chosen by RandomSearch and fivefold cross-validation.
3 RESULTS
3.1 Participant characteristics
During the baseline survey of CHARLS, 17,708 Chinese residents participated, of whom 7681 were equal or older than 60 years old, and 5357 participants had data on at least four components. Participants classified as frailty (n = 407) or containing missing data (n = 1819) were also excluded from the study. Eventually, 3141 residents were included in the study, and 1926 of them were categorized as prefrailty and 1215 were identified as robustness.
3.2 Feature selection
After 318 rounds of RFE (Supporting Information S1: Figure S1), the total number of features utilized in our stacking model dropped from 80 features to 57 features (Supporting Information S1: Table S4). Our results also demonstrated that the task aware RFE methods can significantly decrease the number of features while simultaneously improving the model performance of the stacking model (AUROC: 0.7599 [95% CI: 0.7222, 0.7965] vs. 0.7638 [95% CI: 0.7251, 0.8004]). We also evaluated the performance of individual models including RF, XGBoost, LighGBM, CatBoost. XT, ANN, LR before (Table 1) and after (Table 2) the feature selection and only LR reports a worsen model performance after feature pruning.
| RF | XGBoost | LightGBM | CatBoost | XT | ANN | LR | Stacking | Distilled CatBoost | |
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | 0.6864 | 0.6805 | 0.6732 | 0.6965 | 0.6599 | 0.6209 | 0.6574 | 0.7159 | 0.6968 |
| [0.6491, 0.7241] | [0.6443, 0.7177] | [0.6363, 0.7113] | [0.6587, 0.7321] | [0.6220, 0.6970] | [0.5837, 0.6603] | [0.6204, 0.6938] | [0.6794, 0.7528] | [0.6603, 0.7337] | |
| AUROC | 0.7292 | 0.7380 | 0.7127 | 0.7492 | 0.7103 | 0.6347 | 0.6792 | 0.7599 | 0.7532 |
| [0.6882, 0.7669] | [0.6979, 0.7764] | [0.6690, 0.7526] | [0.7095, 0.7857] | [0.6693, 0.7508] | [0.5906, 0.6787] | [0.6353, 0.7191] | [0.7222, 0.7965] | [0.7142, 0.7886] | |
| F1 Score | 0.7831 | 0.7540 | 0.7777 | 0.7646 | 0.7715 | 0.7028 | 0.7507 | 0.7865 | 0.7733 |
| [0.7522, 0.8116] | [0.7193, 0.7869] | [0.7467, 0.8079] | [0.7309, 0.7972] | [0.7414, 0.8021] | [0.6633, 0.7399] | [0.7168, 0.7814] | [0.7531, 0.8167] | [0.7412, 0.8033] | |
| Precision | 0.6956 | 0.7342 | 0.6821 | 0.7499 | 0.6703 | 0.6965 | 0.6949 | 0.7488 | 0.7331 |
| [0.6544, 0.7343 | [0.6905, 0.7786] | [0.6425, 0.7216] | [0.7087, 0.7928] | [0.6292, 0.7122] | [0.6517, 0.7438] | [0.6509, 0.7382] | [0.7075, 0.7895] | [0.6902, 0.7729] | |
| Recall | 0.8964 | 0.7753 | 0.9051 | 0.7804 | 0.9093 | 0.7097 | 0.8167 | 0.8288 | 0.8186 |
| [0.8626, 0.926] | [0.7343, 0.8158] | [0.8735, 0.9355] | [0.7393, 0.8198] | [0.8782, 0.935] | [0.6650, 0.7532] | [0.7763, 0.8561] | [0.7906, 0.8656] | [0.7815, 0.8553] |
- Abbreviation: AUROC, area under the receiver operator characteristic curve.
- Note: The p-values for all tests are less than 0.001, indicating the significance of the values highlighted in bold.
| RF | XGBoost | LightGBM | CatBoost | XT | ANN | LR | Stacking | Distilled CatBoost | |
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | 0.6915 | 0.6853 | 0.6653 | 0.7125 | 0.6694 | 0.6395 | 0.6446 | 0.7203 | 0.7174 |
| [0.6539, 0.7273] | [0.6475, 0.7193] | [0.6268, 0.7018] | [0.6762, 0.7480] | [0.6316, 0.7065] | [0.6028, 0.6762] | [0.6061, 0.6826] | [0.6842, 0.7576] | [0.6810, 0.7528] | |
| AUROC | 0.7448 | 0.7489 | 0.7152 | 0.7537 | 0.7225 | 0.6662 | 0.6770 | 0.7638 | 0.7560 |
| [0.7057, 0.7828] | [0.7099, 0.7855] | [0.6738, 0.7536] | [0.7152, 0.7911] | [0.6820, 0.7621] | [0.6238, 0.7083] | [0.6328, 0.7173] | [0.7251, 0.8004] | [0.7169, 0.7928] | |
| F1 Score | 0.7860 | 0.7637 | 0.7358 | 0.7844 | 0.7765 | 0.7168 | 0.7414 | 0.7906 | 0.7884 |
| [0.7539, 0.8156] | [0.7310, 0.7948] | [0.6992, 0.7692] | [0.7525, 0.8144] | [0.7448, 0.8054] | [0.6803, 0.7518] | [0.7077, 0.7738] | [0.7581, 0.8217] | [0.7555, 0.8172] | |
| Precision | 0.6998 | 0.7266 | 0.7341 | 0.7455 | 0.6779 | 0.7117 | 0.6863 | 0.7503 | 0.7483 |
| [0.6596, 0.7402] | [0.6839, 0.7689] | [0.6889, 0.7767] | [0.7037, 0.7878] | [0.6370, 0.7181] | [0.6658, 0.7551] | [0.6423, 0.7288] | [0.7061, 0.7922] | [0.7057, 0.7888] | |
| Recall | 0.8967 | 0.8054 | 0.7381 | 0.8282 | 0.9092 | 0.7225 | 0.8067 | 0.8359 | 0.8334 |
| [0.8646, 0.9239] | [0.7674, 0.8444] | [0.6925, 0.7789] | [0.7876, 0.8653] | [0.8772, 0.9361] | [0.6780, 0.7685] | [0.7649, 0.8435] | [0.7980, 0.8727] | [0.7961, 0.8663] |
- Note: The p-values for all tests are less than 0.001, indicating the significance of the values highlighted in bold.
3.3 Model performance
In the current study, stacking model outperforms all individual model before (AUROC: 0.7599 [95% CI: 0.7222, 0.7965]) and after (AUROC: 0.7638 [95% CI: 0.7251, 0.8004]) the RFE. Our result demonstrates that despite the highest AUROC is obtained by Catboost among all individual models, the stacking-CatBoost distillation module can still further improve the model performance with a statistic difference (p = 0.005). Eventually, the CatBoost model reaches a similar model performance compared with stacking model, while significantly reducing the inference time (Supporting Information S1: Figure S2).
3.4 Model explanation
Figure 2a presents a SHAP summary plot, which illustrates the magnitude, prevalence, and direction of the top 10 features with the greatest impact on the distilled CatBoost model. The living city, BMI, and peak expiratory flow (PEF) were identified as the three most significant contributors to the risk of pre-frailty.
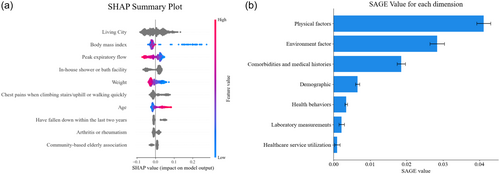
SHAP values also indicate the associations between features and the pre-frailty risk (Supporting Information S1: Figure S4). Living in certain cities and lacking community-based older adults associations increase pre-frailty risk. Conversely, living in a house with a household-installed water heater, and being younger with higher BMI, PEF, and weight decrease pre-frailty risk. Chest pains during physical exertion fall within the last 2 years, and arthritis or rheumatism diagnoses are the top three comorbidities that increase pre-frailty risk.
Figure 2b illustrates the contribution of various dimensions to the model performance of the distilled CatBoost. Physical factors, including BMI, PEF, weight, height, DBP, SBP, and pulse, are identified as the most impactful dimension, while healthcare service utilization, such as outpatient and hospitalization reasons, is found to be the least impactful dimension.
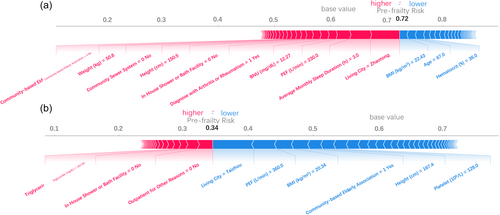
3.5 Interpretable individual pre-frailty scores
As shown in Figure 3, the PACIFIC framework can visualize personal risk using features with the help of TreeExplainer and force plot. Figure 3a shows a pre-frail individual with a pre-frailty risk score of 0.72. Factors such as living in Zhaotong, a relatively underdeveloped mountainous city in southwestern China, without community-based older adult associations, community sewer systems, or in-house shower/bath facilities, as well as short average monthly sleep duration (3 h), relatively low PEF (230 L/min), height (150.5 cm), weight (50.8 kg), and BUN (12.27 mg/dL) significantly increased the risk despite the individual's relatively young age (67), ideal BMI (22.43 kg/m2), and hematocrit (36%). Figure 3b shows a robust individual with a pre-frailty risk score of 0.34. Factors such as living in Taizhou, a relatively developed coastal city in southeastern China, with community-based older adult associations and ideal PEF (360 L/min), BMI (20.34 kg/m2), height (167.4 cm), and platelet count (128 × 109/L decreased the risk, while outpatient treatment, relatively high triglyceride levels (162.84 mg/dL), and the absence of in-house shower/bath facilities increased the risk.
4 DISCUSSION
In our study, we established an explainable machine learning framework (PACIFIC) to identify pre-frail older adults living in the community using the information on laboratory measurements, physical factors, demographics, health behaviors, comorbidities and medical histories, healthcare service utilization, household facilities, and neighborhood resources. This represents the first attempt to integrate state-of-the-art machine learning techniques and explanation methods to systematically study pre-frailty. We have enhanced the practical value of our study by introducing an easy-to-use and explainable pre-frailty risk calculator.
Our study demonstrates that distilled Catboost is an effective ML model for identifying pre-frailty from community-dwelling older adults. In our study, distilled Catboost surpassed all other individual models. Only a few studies [7, 8] have explored the feasibility of identifying pre-frailty from community-dwelling old adults using ML. We significantly improved the model performance (AUROC: 0.7560 vs. 0.7220 [7]) using the PACIFIC framework by leveraging the advantages of CatBoost and KD. Gradient boosting decision tree algorithms are known to efficiently model the heterogeneous data [28]. Among these models, CatBoost outperforms others [20, 28], due to the its build-in ordered target statistic [20] that effectively processes categorical features common in modern data-driven medical problems [29]. We also utilized KD to boost our model performance. Although stacking models [23] can significantly reduce variance and improve performances, it increases inference time and is difficult to deploy. KD [21] can bridge the knowledge gap between stacking (teacher) and individual (student) models (Supporting Information S1: Figure S3), trading off between model performance and inference time (Supporting Information S1: Figure S2).
Machine learning models such as distilled CatBoost provide superior risk estimates, but the source of risk is often non-interpretable [17]. To overcome this issue, we visualized global and individual risk sources using SHAP summary plot (Figure 2a) and SHAP dependence plot (Supporting Information S1: Figure S4), making our study one of the most comprehensive clinical applications with SHAP values. We identified the top 10 most impactful features and their associations with pre-frailty risk, most of which have been reported by prior epidemiological studies, including BMI [30, 31], PEF [32, 33], weight [34], age [35], falls within the last 2 years [36], and arthritis or rheumatism [37, 38] (Supporting Information S1: Figure S3). However, their complex interactions result in varying risk contributions. With the aid of a SHAP individual explanation plot (Figure 3), WE can visually discern the risk contributions of each feature, helping individuals improve their self-awareness [19] or providing potential avenues for risk modification [39].
We employed feature selection to effectively eliminate less predictive variables and methodically examine potential pre-frailty risk factors from various sources. This led to the identification of previously overlooked risk factors, including chest pains during physical exertion, the presence of in-house shower or bath facilities, and participation in community-based older adult associations. Chest pains during physical exertion have been recognized as a significant indicator of acute coronary syndrome [40], which is closely linked to pre-frailty [41]. However, the connection between chest discomfort during physical activity and frailty remains unclear. Meanwhile, few studies have explored the determinants of household amenities and neighborhood resources [42]. Our research revealed that the absence of in-home shower or bath amenities or community-based senior citizen organizations increased the risk of pre-frailty. The absence of in-home shower or bath amenities may hinder personal hygiene or reflect substandard indoor conditions, increasing the risk of pre-frailty. On the other hand, senior citizen organizations offer opportunities for social interaction [43], physical exercise [44], and access to health information and resources [45], aiding in the prevention of pre-frailty. From a global standpoint, our study highlighted the significance of environmental factors using SAGE values (Figure 2b), which provide comparable contributions that aid in model explanation and knowledge discovery in clinical practice [46, 47]. Our findings align with recent arguments that environmental factors play a crucial role in health longevity [45] and frailty [42, 48].
One major drawback of traditional epidemiological research is the difficulty of modeling high cardinality features, which are frequently one-hot encoded (dummy) into sparse vectors [25], leading to an underestimate of their importance. However, the build-in algorithm of CatBoost efficiently handles these features [20] and, in the PACIFIC framework, ranked living city as the most influential feature (Figure 2a) in contributing to the pre-frailty risk through geographic variations. This finding aligns with previous studies [15, 49] that reported regional disparities in healthcare as a cause for geographic variation in the prevalence of frailty among Chinese community-dwelling older adults. This could be attributed to several underlying factors, such as geographic factors (i.e., altitudes and climates) [50], imbalanced healthcare facilities, and service of different cities [51], as well as varying average city education and income level [52]. Our findings underscore the importance of considering geographical variations in public health strategies and interventions aimed at reducing pre-frailty among older adults.
To our knowledge, this was also the first study using a machine learning-based method to summarize the dimensional risk based on sources of risk. The SAGE values enabled us to visualize the global impact of feature dimension and quantify their importance. This is critical not only for clinical counseling but also for the development of optimized preventative strategies that are personalized and standardized. The early identification of pre-frailty can significantly reduce the onset of frailty and reduce healthcare costs. There is emerging evidence that indicates early prevention needs more person-centered community care planning [53]. It is also known that these older adults represent a heterogeneous cohort, and a one-size-fits-all approach is unlikely to be successful. For practice, a comprehensive assessment could be distilled to a 4-layer system: environmental factors, social factors, clinical factors, and biological factors. This approach combines system-level targets with the development of person-centered healthcare, which has been little studied. Our model may provide earlier alerts compared to commonly used assessment tools, such as Activities of Daily Living evaluations and serve as a valuable self-assessment or clinical decision support tool. The integration of our model with existing frameworks could enhance its clinical utility and facilitate broader adoption. Future work is needed to validate the effectiveness of our frameworks.
The best timing for using ML as a clinical decision-support tool might be embedded within the physical examination and outpatient services, for example, where an on-screen notification during a consultation informs the clinician about their patient's pre-frailty risk. These notifications might be particularly beneficial in health-care settings in which a patient does not always see the same clinician, and the clinician might not be aware of a patient's full history and living environment.
Our research has several limitations. First, although BMI is an important criterion in the PFP, we did not exclude it due to its cost-efficiency and our aim to analyze the effect of high BMI on the risk of pre-frailty. Second, due to the lack of information on shrinking [54] and neighborhood information [42], we only analyzed the baseline data, which introduces potential bias and limits our study to a cross-sectional analysis, despite our framework's potential for extension by modifying the outcome. Third, although we have rigorously validated the model performance on a holdout unseen set, external validations are still required to ensure the robustness of our model. Fourth, we included 80 features from seven dimensions based on existing literature and experts' consultation, the list is not exhaustive. Meanwhile, the relationship detected by our study cannot be claimed to be causal, and further studies are required. Nonetheless, the study demonstrated the utility of the state-of-the-art ML and explanation method in accurate and interpretable pre-frailty risk quantification which may facilitate the risk stratification and residency consultation. The identification and understanding of several underappreciated determinants in our framework may also have important implications in policymaking and intervention design.
5 CONCLUSIONS
In conclusion, we have developed an accurate and interpretable pre-frailty risk assessment framework using state-of-the-art ML techniques and explanation methods. Our framework incorporates a wide range of features and determinants, allowing for a comprehensive and nuanced understanding of pre-frailty risk. The results of our study demonstrate the potential for ML-based approaches to improve risk stratification and inform targeted interventions in the field of epidemiology. Beyond the current study, the PACIFIC framework holds promise for adaptation to other populations and health conditions, thereby contributing to the formulation of broader public health strategies. Further research is needed to validate our findings and explore the potential for wider application of our framework.
AUTHOR CONTRIBUTIONS
Chenlin Du: Conceptualization (equal); data curation (equal); software (equal), validation (equal); visualization (equal); writing—original draft (equal). Zeyu Zhang: Conceptualization (equal). Baoqin Liu: Investigation (equal). Zijian Cao: Investigation (equal). Nan Jiang: Writing—original draft (equal); writing—review & editing (equal). Zongjiu Zhang: Funding acquisition (equal); project administration (equal); supervision (equal).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
Professor Zongjiu Zhang is a member of the Health Care Science Editorial Board. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
The study protocol was approved by the Ethical Review Committee at Peking University (IRB00001052−11015).
INFORMED CONSENT
All participants were provided with written consent.
Open Research
DATA AVAILABILITY STATEMENT
No new data are generated in this study. The CHARLS date is available at http://charls.pku.edu.cn/.




