Phenolic composition and antioxidant activity of Southern Italian monovarietal virgin olive oils
Abstract
The phenolic composition and antioxidant activity of several monovarietal extra virgin olive oils used as blenders for the production of Collina di Brindisi protected designation of origin (PDO) oil, produced between December 2008 and January 2009 using two-phases or three-phases extraction system, were evaluated and compared with other manufacturer products designated as PDO. Oils were taken from the most representative ones industrial oil mills in the PDO geographical area. The parameters assessed were free acidity, peroxide value, K232 and K270 indices, organoleptic characteristics, total phenolic content (TPC), phenolic profile, and antioxidant activity coefficient (AAC). The phenolic contents and profiles of the monovarietal oils showed remarkable differences with respect to PDO oils. The variables that exerted a major influence on phenols concentration were the maturity degree of olives (December>January), followed by the extraction system (two-phase>three-phase), and place of growing. The Pearson r correlation index showed that AAC was positively correlated with TPC, p-coumarate, and 3,4-DHPEA-EA, and negatively correlated with peroxide value.
Practical applications: The results provide detailed information about: (i) the phenolic composition and the AAC of several monovarietal extra virgin olive oils used as blenders for the production of a PDO oil; (ii) the impact of genetic variability, place of growing, olive maturity degree, and extraction technology on oil phenol compounds; and (iii) the relationships among each phenolic compound and AAC, and their potential utilization as analytical index of antioxidant activity. It is important to study the phenolic compounds and antioxidant activity of monovarietal extra virgin olive oils used to produce PDO oil and to compare with the relative PDO samples in order to define a possible analytical tool able to verify what is stated in the label for consumer information and protection.
Abbreviations:
AAC, antioxidant activity coefficient; AcPin, (+)-1-acetoxypinoresinol; p-Ca, p-coumaric acid; 3,4-DHPEA-AC, hydroxytyrosol acetate; 3,4-DHPEA-EA, oleuropein aglycone; 3,4-DHPEA-EDA, decarboxymethyl oleuropein aglycone; p-HPEA-EA, ligstroside aglycone; p-HPEA-EDA, decarboxymethyl ligstroside aglycone; HPin, hydroxypinoresinol; PDO, protected designation of origin; Pin, pinoresinol; PV, peroxide value; TPC, total phenolic content
Introduction
Virgin olive oil is the basis of the Mediterranean diet. It is obtained from the olive tree fruits solely by mechanical or other physical means without any treatment other than washing, decantation, centrifugation, or filtration 1. Olive oil is rich in MUFA, EFA, and nutritionally valuable minor polar compounds such as phenols. Although phenols are minor constituents the assessment of their content and profile is extremely important in the study of nutraceutical properties of olive oil. The amount of phenols depends on several factors including cultivar, agronomic techniques, olive ripening stage, fruit pre-storage, extraction technologies, and time and storage conditions of the final product 2-7. The most important phenols in virgin olive oil are the series of benzoic acid derivatives (3-hydroxybenzoic, p-hydroxybenzoic, 3,4-dihydroxybenzoic, gentisic, vanillic, gallic, and syringic), cinnamic acids and derivatives (o-coumaric, p-coumaric, caffeic, ferulic, and sinapic), phenyl ethyl alcohols (tyrosol and hydroxytyrosol), other phenolic acids [p-hydroxyphenylacetic, 3,4-dihydroxyphenylacetic, 4-hydroxy-3-methoxyphenylacetic, and 3-(3,4-dihydroxyphenil)propanoic], dialdehydic forms of secoiridoids (decarboxymethyloleuropein aglycone or 3,4-DHPEA-EDA and decarboxymethyl ligstroside aglycon or p-HPEA-EDA), secoiridoid aglycones (oleuropein aglycone or 3,4-DHPEA-EA, ligstroside aglycone or p-HPEA-EA, aldehydic form of oleuropein aglycone, and aldehydic form of ligstroside aglycone), flavonols [(+)-taxifolin], flavones (apigenin and luteolin), lignans [(+)-pinoresinol, (+)-1-acetoxypinoresinol, and (+)-1-hydroxypinoresinol], and hydroxyisochromans [1-phenyl-6,7-dihydroxyisochroman and 1-(3′-methoxy-4′-hydroxy)phenyl-6,7-dihydroxyisochroman] 8. These compounds are known to possess antioxidant properties that delay the oxidation of the oil and also play an important role in the primary and secondary prevention of some major diseases such as cardiovascular 9, cancer 10, premature aging 11, degenerative diseases of the nervous system 12, and spermatogenesis 13. Recently, the use of an emulsifier to stabilize the phenolic compounds added in the preparation of a nutraceutical olive oil in order to extend the shelf life has been evaluated 14. Results showed that the enriched oils presented higher oxidative stability values and higher total phenolic contents (TPC) than the control, and the use of lecithin as emulsifier limited the increase of bitterness. Several studies have assessed the relationship between phenols concentration, particularly the secoiridoid derivatives such as 3,4-DHPEA-EA, 3,4-DHPEA-EDA, and p-HPEA-EDA, and bitter and spicy taste in virgin olive oil 15-19.
Among the Italian protected designation of origin (PDO) extra virgin olive oils, Collina di Brindisi oil (Apulia Region, Southern Italy) is characterized by an equilibrate taste, in particular fruity, bitter, and spicy. It is obtained by blending at least 70% of Ogliarola cultivar and 30% of other varieties such as Carolea, Cima di Melfi, Coratina, Frantoio, Leccino, Nociara, Picholine, and other unspecified Olea europaea varieties that are grown in the production area 20, 21. Assessing the full chemical composition of products sold with a specific label is essential in order to guarantee their quality and authenticity and in doing so to safeguard consumers from commercial frauds. The effectiveness of microsatellite DNA markers and of some quality chemical indices in checking the identity of Collina di Brindisi PDO was investigated by Pasqualone et al. 22. They found that the examination of a limited number of DNA microsatellites enables the identification of the Ogliarola salentina cultivar in this PDO oil. On the other hand, several authors have demonstrated that phenolic profile may represent a further parameter to be considered when studying quality and authenticity of monovarietal and PDO oils 23-25. To the best of our knowledge, little information is available on the identities of the phenol compounds of monovarietal extra virgin olive oils from cultivars that constitute PDO Collina di Brindisi.
The goal of this study was firstly to evaluate the antioxidant activity coefficient (AAC) of monovarietal extra virgin olive oils used as blenders for the production of Collina di Brindisi PDO because this parameter is not only strongly linked to shelf life of the product but have undoubted advantages on consumers health, as widely reported in literature. In addition, a study was carried out to understand which of the phenolic substances present in the oils are more related to antioxidant activity in order to characterize this important PDO oils with the knowledge of new analytical parameters indicators of antioxidant activity.
Materials and methods
Olive oil samples
A total of 19 extra virgin olive oils obtained from oil mill industries located in the Brindisi district that received an European PDO (Fig. 1) were considered during the harvesting period between December 2008 and January 2009. Samples (Table 1) were collected from reliable oil mills and comprised 15 different monovarietal oils of cultivars belonging to Collina di Brindisi PDO and 4 authentic (i.e., of guaranteed provenance) PDO oils from different producers associated to the Collina di Brindisi consortium. For each monovarietal and PDO sample three sub-samples were taken. The sub-sample was a mix of aliquots taken from the top, the middle and the bottom of a 15 tons tank. Each sub-sample was analyzed in triplicate. Results in the tables are the average with SD of 9 determinations (3 sub-samples × 3 replicates = 9 analyses). Oils were placed in 5-L cans and 1 wk later (time required to natural decanting) were transferred in 1 L dark glass bottles with a headspace of about 1% of total volume, and stored in the dark at RT until analysis.
Geographical area of PDO Collina di Brindisi extra virgin olive oil.
| Code | Cultivar | Origin | Harvest datea); blendb) production | Technology |
|---|---|---|---|---|
| CA | Carolea | Fasano | January 2009 | Two-phase |
| CM1 | Cima di Melfi | Ostuni | December 2008 | Three-phase |
| CM2 | Cima di Melfi | Fasano | December 2008 | Two-phase |
| CO1 | Coratina | Ostuni | December 2008 | Two-phase |
| CO2 | Coratina | San Vito dei Normanni | January 2009 | Two-phase |
| CO3 | Coratina | Fasano | January 2009 | Two-phase |
| FR1 | Frantoio | Ostuni | December 2008 | Two-phase |
| FR2 | Frantoio | Ostuni | January 2009 | Two-phase |
| LC | Leccio del Corno | Ostuni | January 2009 | Two-phase |
| LE | Leccino | Ostuni | December 2008 | Two-phase |
| OG | Ogliarola | Fasano | December 2008 | Two-phase |
| NO | Nociara | Ostuni | January 2009 | Three-phase |
| PI1 | Picholine | Ostuni | December 2008 | Two-phase |
| PI2 | Picholine | San Vito dei Normanni | December 2008 | Two-phase |
| PI3 | Picholine | Ostuni | January 2009 | Three-phase |
| PDO1 | Ogliarola (70)c), Leccino (20), Cima di melfi, Coratina, Frantoio and Picholine (10) | San Vito dei Normanni | February | Two-phase |
| PDO2 | Ogliarola (70), Leccino (10), Frantoio (8), Coratina (5), Cima di Melfi (5) and Picholine (7) | Ostuni | February | Three-phase |
| PDO3 | Ogliarola (70), Leccino (15), Coratina (8) and Picholine (7) | Ostuni | February | Two-phase |
| PDO4 | Ogliarola (89) and Cima di Melfi (11) | Fasano | February | Two-phase |
- a) Monovarietal oils.
- b) PDO oils.
- c) Proportion.
Determination of quality indices
Acidity (expressed as percentage of oleic acid), peroxide value (PV, as meqO2/kg), spectrophotometric indices K232 and K270, and sensory score were determined according to the analytical methods described in the European Union standard methods 26 and subsequent amendments.
Determination of phenolic compounds
Phenolic compound extraction
Phenols were extracted from extra virgin olive oils by liquid–liquid partition using a mixture of methanol/water (70:30 v/v) according to procedure reported by Gambacorta et al. 5. Briefly, 2 mL of hexane and 2 mL of methanol/water mixture were added to 5 g of olive oil sample and mixed with a vortex for 10 min. The hydro-methanolic phase containing phenols was separated from the oily phase by centrifugation (6000 × g, 4°C, 10 min), recovered, and subjected to further centrifugation (13 000 × g, at RT, 4 min). Finally, phenol extract was recovered and then filtered through a 0.45 µm nylon filter (DISMIC-13NP, Advantec, Toyo Roshi Kaisha, Tokjo, Japan) before analysis. The extracts for HPLC analysis were obtained according to the same protocol, and 0.5 mL solution of gallic acid at a concentration of 100 mg/mL, prepared in the same methanol/water mixture used for phenols extraction, was added as internal standard (Extra-synthese, Genay Cedex, France).
Total phenolic compounds
The determination of the total phenolic compounds included the use of the Folin–Ciocalteu reagent and the method was adapted from Di Stefano et al. 27. In a test tube, 100 µL of phenolic extract or phenolic standard were mixed with 100 µL of Folin–Ciocalteu reagent and, after 4 min, with 800 µL of Na2CO3 (5%). The mixture was then heated in a 40°C water bath for 20 min and the total phenol content was determined by colorimetrically at 750 nm. The standard curve was prepared using diluted solutions of gallic acid in methanol/water mixture. The TPC was expressed as gallic acid equivalents (mg/kg).
HPLC-ESI-MS phenolic profile
The analysis of phenolic profile of extracts was carried out using an Agilent HPLC 1100 capillary system consisting of a G1376A quaternary pump, G1379A degasser, G1377A auto-sampler injector (3-µL sample loop), G1315C diode array detector (DAD), and Trap XCT plus MS detector (MSD) (Agilent technologies, Santa Clara, CA, USA) in a series configuration. Separation of phenol compounds was performed by a NovaPack C18 column (150 × 2.1 mm id, 4 µm particle size, Waters, Milford, MC, USA) using the mobile phase consisting of (A) water/acetic acid (98:2 v/v) and (B) methanol/acetonitile (1:1 v/v) at a constant flow rate of 0.2 mL/min. The gradient program was as follows: 0–30 min 100% A; 30–45 min 70% A; 45–55 min 50% A; 55–65 min 40% A; 65–75 min 0% A. DAD was monitored at 280 nm, and spectra were recorded at wavelength range 240–380 nm. MSD was an ion trap mass spectrometer equipped with an ESI source. The operative conditions and the analysis procedure of ESI-MS were the same reported in Gambacorta et al. 5. All data were acquired and processed using the ChemStation software (Agilent Technologies). Identification of phenolic compounds was achieved by combining elution pattern, UV–Vis and MS spectra, low-energy collision induced tandem MS CID-MS/MS fragmentation patterns, and data found in the literature 23, 28, 29. Quantitative analysis was performed by the method of the internal standard (gallic acid) and determining the response factors taking into account the recovery percentages of the phenolic compounds and the internal standard 30.
Determination of antioxidant activity
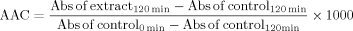
The AAC can assume values between 0 (total absence of antioxidant activity) and 1000 (maximum antioxidant activity obtained when whole the β-carotene was preserved from the oxidation). Analyses were carefully performed to avoid heavy metals contamination that acts as prooxidant.
Statistical analysis
Analyses were repeated at least three times for each sample. Data were expressed as the mean ± SD of three-independent experiments and analyzed for correlation, linear regression, and comparative ANOVA using SPSS v18.0 Statistics Software (SPSS, Chicago, IL). The criterion for significance was set at p<0.05.
Results and discussion
Quality indices
According to EU Commission Regulation 1989 33, a virgin olive oil is defined as extra category if it conforms to a series of chemical and sensory parameters such as free fatty acid (≤0.8 oleic acid/100 g oil), peroxide value (≤20 meqO2/kg), K232 (≤2.50), K270 (≤0.22), and median of defects = 0 and median of fruity>0. In Table 2 qualitative indices and antioxidant activity of all samples are reported. As values show, all oils can be defined as extra virgin olive oils. Regarding the sensory tests, all samples had the median of defects equal to zero and the median of fruity greater than 3.0, values indicatives of products with good sensory characteristics. The variability of the quality indices values among the monovarietal samples was higher than those observed for the four PDO oils. In particular, 11 monovarietal oils out of 15 (about 73%) exhibited an acidity lower than 0.50 g/100 g, which is below that required for an oil to be considered of high quality, whereas PDO oils showed slightly higher values (0.53–0.70 g/100 g). Concerning the primary products of oxidation (hydroperoxides), about 73% of the monovarietal samples were not minimally oxidized showing a peroxide value of lower than 10 meqO2/kg. Similar values were found in PDO oils (8–10 meqO2/kg). The value of a further parameter related to hydroperoxides, K232, in monovarietal oils ranged from 1.56 to 2.09, whereas in PDO oils it was ≥2.00. Regarding the secondary products of oxidation, the K270 value of monovarietal oils ranged from 0.099 to 0.179, whereas for PDO oils it was from 0.131 to 0.159. All samples analyzed, particularly monovarietal oils, exhibited a very high variability in TPC, showing that phenols are strongly affected by the cultivar, to confirm what recently reported in a review on hydrophilic antioxidants of virgin olive oil by El Riachy et al. 34. The highest TPCs were detected for Picholine and Coratina, followed by Frantoio, Leccio del Corno and Ogliarola, whereas the lowest ones were found for Carolea and Nociara. Data shown in Table 2 indicated that TPC was more closely correlated to olives ripeness than to place of growing (PG) and extraction system. In fact, oils of the same cultivar produced in December showed higher TPCs than those produced in January (CO1 > CO2 = CO3), (F1>F2) and (P1 > P2 ≈ P3). As expected, both monovarietal and PDO oils were characterized by a great variability in antioxidant activity. Among the monovarietal samples, the AAC ranged from 73 to 428. These values were found in Frantoio oils produced in Ostuni by employing a two-phases decanter from olives harvested in December and January, respectively. This result highlights that Frantoio grown in Apulia suffered a dramatic drop in the phenol compounds during olive maturation and, consequently, the oil antioxidant activity decreased.
| Sample | Acidity (g/100 g) | PV (meqO2/kg) | K232 | K270 | TPC (mg/kg) | AAC |
|---|---|---|---|---|---|---|
| CAa) | 0.31 ± 0.04 | 11.82 ± 0.01 | 1.91 ± 0.07 | 0.109 ± 0.008 | 148 ± 6 | 187 ± 61 |
| CM1 | 0.25 ± 0.04 | 15.17 ± 0.57 | 1.68 ± 0.02 | 0.107 ± 0.018 | 177 ± 2 | 154 ± 25 |
| CM2 | 0.56 ± 0.08 | 9.38 ± 0.66 | 1.79 ± 0.05 | 0.141 ± 0.027 | 170 ± 6 | 238 ± 44 |
| CO1 | 0.34 ± 0.00 | 8.16 ± 0.62 | 1.66 ± 0.02 | 0.153 ± 0.008 | 420 ± 10 | 225 ± 55 |
| CO2 | 0.22 ± 0.00 | 9.13 ± 0.56 | 2.05 ± 0.01 | 0.155 ± 0.018 | 285 ± 8 | 178 ± 42 |
| CO3 | 0.51 ± 0.00 | 9.43 ± 0.61 | 1.62 ± 0.04 | 0.152 ± 0.018 | 293 ± 9 | 178 ± 39 |
| FR1 | 0.28 ± 0.00 | 6.30 ± 0.55 | 1.75 ± 0.03 | 0.121 ± 0.023 | 315 ± 9 | 428 ± 34 |
| FR2 | 0.51 ± 0.00 | 12.85 ± 0.15 | 1.74 ± 0.01 | 0.087 ± 0.000 | 152 ± 4 | 73 ± 27 |
| LC | 0.31 ± 0.04 | 4.60 ± 0.55 | 1.56 ± 0.01 | 0.120 ± 0.008 | 294 ± 6 | 284 ± 24 |
| LE | 0.17 ± 0.00 | 6.21 ± 0.61 | 1.62 ± 0.02 | 0.099 ± 0.013 | 186 ± 5 | 169 ± 14 |
| OG | 0.65 ± 0.04 | 3.98 ± 1.74 | 1.98 ± 0.04 | 0.179 ± 0.014 | 266 ± 4 | 186 ± 58 |
| NO | 0.23 ± 0.00 | 8.36 ± 0.66 | 1.83 ± 0.00 | 0.101 ± 0.004 | 167 ± 4 | 188 ± 27 |
| PI1 | 0.37 ± 0.04 | 2.95 ± 0.02 | 1.72 ± 0.01 | 0.150 ± 0.008 | 497 ± 9 | 299 ± 57 |
| PI2 | 0.17 ± 0.00 | 10.20 ± 0.60 | 2.09 ± 0.01 | 0.174 ± 0.006 | 298 ± 10 | 222 ± 30 |
| PI3 | 0.45 ± 0.00 | 5.57 ± 0.53 | 1.79 ± 0.02 | 0.149 ± 0.008 | 325 ± 7 | 279 ± 52 |
| PDO1 | 0.56 ± 0.08 | 8.33 ± 0.71 | 2.10 ± 0.15 | 0.154 ± 0.010 | 216 ± 4 | 181 ± 31 |
| PDO2 | 0.64 ± 0.04 | 8.90 ± 0.05 | 2.14 ± 0.02 | 0.131 ± 0.014 | 277 ± 7 | 314 ± 35 |
| PDO3 | 0.70 ± 0.04 | 9.89 ± 0.08 | 2.25 ± 0.01 | 0.159 ± 0.005 | 194 ± 10 | 160 ± 21 |
| PDO4 | 0.53 ± 0.04 | 10.30 ± 0.08 | 1.99 ± 0.03 | 0.142 ± 0.025 | 132 ± 2 | 125 ± 19 |
- PV, peroxide value; TPC, total phenolic content; AAC, antioxidant activity coefficient.
- a) Sample codes are reported in Table 1. Values are expressed as mean ± SD.
Phenolic profile
Figure 2 shows a representative HPLC/DAD profile at 280 nm of the phenolic extract obtained from PDO3 extra virgin olive oil. In Table 3 the phenolic compositions of oils analyzed by HPLC within 3 months from production are reported. Remarkable differences in the phenolic profile were evident among the samples. As expected, a high variability was found for the monovarietal oils, confirming the influence of genotype 4, 6, 30. In order to evaluate the effect of PG, olive maturity index (OMI; December vs. January), and technology (TL; two-phases vs. three-phases decanter), a pair comparison test was applied to the total phenols of Cima di Melfi, Coratina, Frantoio, and Picholine. Results indicated a greater influence of OMI and TL with respect to PG (December>January; two-phase>three-phase); in particular, CM2 oil (Fasano, two-phase) presented a total phenols content about 45% higher than CM1 (Ostuni, three-phase), mainly due to a major presence of 3,4-DHPEA-EDA + hydroxypinoresinol (HPin). These results confirm that oils with higher phenols contents were obtained with a two-phase decanter with respect to a three-phase 35, 36. Similar results were found for Picholine oils of Ostuni. In fact, sample obtained in December with a two-phase system (PI1) showed a phenols content about twice higher than that obtained in January with a three-phase system (PI3). The higher difference in total phenols observed in Ostuni Picholine and Cima di Melfi oils could be explained by the different degree of ripeness of olives (December vs. January for Picholine; December for both Cima di Melfi). The influence of degree of olive ripeness on total phenols was confirmed by comparing Coratina and Frantoio oils produced with the same technology (two-phase) in December and January. On the whole, the highest total phenols contents, particularly 3,4-DHPEA-EDA + HPin (about 40 and 33% of total phenols, respectively), were detected in Coratina and Picholine oils from Ostuni produced in December with a two-phases decanter. On the contrary, the lowest total phenols values were found in Carolea and Frantoio which were characterized by the highest amounts of a acetoxypinoresinol (about 48 and 37% of total phenols, respectively), produced in January with a two-phase system. Among PDO oils, those from Ostuni presented a total phenols content about 40% higher than those from San Vito dei Normanni and Fasano, indicating a clear influence of place of growing. Similar results have been reported by Baiano et al. 30 in Moraiolo, Nociara, and Peranzana cultivated in two different districts of Northern Apulia, Italy.
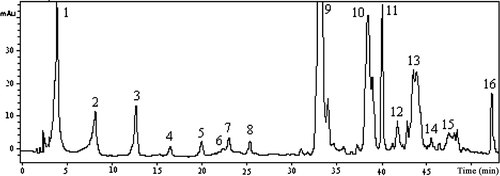
HPLC-DAD profile at 280 nm, obtained from PDO3 oil phenolic extract. (1) Gallic acid (Internal Standard); (2) hydroxytyrosol; (3) tyrosol; (4) vanillic acid; (5) unknown; (6) vanillin; (7) p-coumaric acid; (8) 3,4-DHPEA-AC, hydroxytyrosol acetate; (9) 3,4-DHPEA-EDA + HPin, decarboxymethyl oleuropein + hydroxypinoresinol; (10) p-HPEA-EDA + Pin, decarboxymethyl ligstroside aglycone + pinoresinol; (11) acetoxypinoresinol; (12) lutein; (13) 3,4-DHPEA-EA, oleuropein aglycone; (14) apigenin; (15) p-HPEA-EA, ligstroside aglycone; (16) unknown.
| Sample | HTyr | Tyr | VA | V | p-CA | 3,4-DHPEA-AC | 3,4-DHPEA-EDA + HPin | p-HPEA-EDA + Pin | AcPin | Lut | 3,4-DHPEA-EA | Api | p-HPEA-EA | Total phenols |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAa) | 0.72 ± 0.06 | 1.20 ± 0.04 | 0.57 ± 0.05 | 0.72 ± 0.09 | 1.82 ± 0.08 | 3.67 ± 0.14 | 4.64 ± 0.12 | Nd | 19.73 ± 0.91 | 1.39 ± 0.17 | 4.62 ± 1.11 | 0.76 ± 0.19 | 1.03 ± 0.28 | 40.77 ± 3.10 |
| CM1 | Nd | 0.91 ± 0.05 | 0.27 ± 0.03 | 0.87 ± 0.02 | 0.87 ± 0.16 | Nd | 8.90 ± 0.06 | 21.66 ± 0.51 | 15.15 ± 0.30 | 2.46 ± 0.34 | 6.30 0.28 | 2.01 ± 0.24 | 4.20 ± 0.70 | 63.65 ± 0.60 |
| CM2 | 0.20 ± 0.02 | 1.02 ± 0.08 | 0.19 ± 0.04 | 1.01 ± 0.06 | 1.13 ± 0.09 | 0.36 ± 0.30 | 33.55 ± 0.27 | 23.34 ± 0.89 | 17.45 ± 1.24 | 3.55 ± 0.34 | 5.17 ± 0.55 | 4.09 ± 1.05 | 2.10 ± 0.09 | 93.22 ± 4.87 |
| CO1 | 1.97 ± 0.27 | 1.30 ± 0.03 | 0.19 ± 0.01 | Nd | 1.51 ± 0.41 | 1.68 ± 0.21 | 66.66 ± 2.10 | 47.84 ± 2.61 | 8.80 ± 0.50 | 1.07 ± 0.52 | 28.00 ± 0.61 | Nd | 7.98 ± 0.08 | 166.97 ± 5.33 |
| CO2 | 3.96 ± 0.49 | 4.36 ± 0.04 | 0.22 ± 0.01 | 1.17 ± 0.00 | 1.75 ± 0.60 | 0.43 ± 0.37 | 22.47 ± 1.79 | 25.76 ± 0.11 | 8.37 ± 0.17 | 2.18 ± 0.38 | 4.86 ± 0.29 | 1.79 ± 0.53 | 2.69 ± 0.68 | 80.09 ± 0.64 |
| CO3 | 0.19 ± 0.01 | 0.36 ± 0.12 | Nd | 1.26 ± 0.03 | 1.12 ± 0.04 | 0.62 ± 0.04 | 35.57 ± 0.06 | 30.46 ± 0.11 | 11.48 ± 0.88 | 1.61 ± 0.48 | 5.96 ± 1.00 | Nd | 3.87 ± 1.46 | 92.57 ± 0.30 |
| FR1 | 1.37 ± 0.47 | 1.08 ± 0.04 | 0.17 ± 0.08 | 0.29 ± 0.05 | 0.75 ± 0.09 | 1.03 ± 0.31 | 1.04 ± 0.15 | 30.38 ± 0.42 | 23.29 ± 1.32 | 1.69 ± 0.53 | 1.35 ± 0.41 | 2.02 ± 0.05 | 6.75 ± 0.18 | 71.28 ± 0.08 |
| FR2 | 0.72 ± 0.23 | 2.22 ± 0.03 | 0.55 ± 0.05 | 0.47 ± 0.03 | 0.24 ± 0.05 | 1.18 ± 0.04 | 4.87 ± 0.01 | 9.03 ± 0.22 | 15.17 ± 0.56 | 1.47 ± 0.05 | 3.57 ± 0.18 | 0.49 ± 0.01 | 1.20 ± 0.01 | 41.17 ± 1.49 |
| LC | 0.38 ± 0.20 | 0.53 ± 0.03 | 0.15 ± 0.00 | Nd | 3.44 ± 0.08 | 0.95 ± 0.06 | 27.91 ± 0.31 | 19.69 ± 0.50 | 10.95 ± 1.63 | 2.93 ± 0.09 | 5.88 ± 0.82 | 2.45 ± 0.37 | 1.71 ± 0.21 | 77.03 ± 3.10 |
| LE | 0.53 ± 0.31 | 0.76 ± 0.13 | 0.25 ± 0.04 | Nd | 1.25 ± 0.15 | 1.64 ± 0.19 | 1.56 ± 0.16 | 24.65 ± 2.33 | 25.98 ± 2.43 | 1.54 ± 0.20 | 3.14 ± 0.13 | 3.41 ± 0.48 | 2.12 ± 0.39 | 66.90 ± 6.03 |
| OG | 0.63 ± 0.05 | 1.30 ± 0.08 | 0.35 ± 0.05 | 1.20 ± 0.20 | 1.38 ± 0.05 | 0.63 ± 0.10 | 35.12 ± 0.24 | 20.05 ± 0.19 | 11.83 ± 0.10 | 3.50 ± 0.23 | 17.13 ± 1.63 | 2.66 ± 0.71 | 1,98 ± 0.12 | 97.84 ± 0.87 |
| NO | Nd | 0.58 ± 0.02 | Nd | Nd | 1.74 ± 0.02 | Nd | 8.38 ± 0.52 | 44.32 ± 0.84 | 3.58 ± 0.37 | 1.62 ± 0.67 | 1.44 ± 0.32 | 0.52 ± 0.31 | 1.15 ± 0.27 | 63.39 ± 1.59 |
| PI1 | 1.37 ± 0.26 | 2.25 ± 0.14 | 0.23 ± 0.10 | Nd | 2.78 ± 0.59 | Nd | 44.24 ± 2.64 | 33.75 ± 1.22 | 4.29 ± 0.00 | 1.82 ± 0.76 | 33.03 ± 0.03 | 1.77 ± 0.91 | 8.78 ± 1.26 | 134.35 ± 1.36 |
| PI2 | 2.07 ± 0.46 | 3.52 ± 0.08 | 0.35 ± 0.02 | 1.60 ± 0.15 | 2.47 ± 0.51 | Nd | 17.87 ± 0.60 | 23.38 ± 0.94 | 6.83 ± 0.17 | 2.25 ± 0.95 | 6.10 ± 1.05 | 1.40 ± 0.69 | 4.22 ± 0.77 | 72.12 ± 5.27 |
| PI3 | 0.27 ± 0.06 | 1.04 ± 0.12 | 0.05 ± 0.01 | Nd | 3.66 ± 0.00 | Nd | 23.93 ± 1,46 | 15.61 ± 1.46 | 3.02 ± 0.97 | 2.32 ± 0.08 | 17.18 ± 0.98 | 2.25 ± 0.88 | 3.04 ± 1.06 | 72.41 ± 6.83 |
| PDO1 | 1.01 ± 0.16 | 1.19 ± 0.06 | 0.15 ± 0.03 | Nd | Nd | 0.98 ± 0.15 | 13.56 ± 0.24 | 27.92 ± 0.29 | 4.70 ± 0.81 | 2.13 ± 0.07 | 9.32 ± 0.31 | 1.07 ± 0.03 | 2.44 ± 0.08 | 66.43 ± 2.07 |
| PDO2 | 4.12 ± 0.64 | 3.11 ± 1.52 | 0.64 ± 0.32 | 1.25 ± 0.11 | 2.38 ± 1.02 | 0.90 ± 0.03 | 38.55 ± 2.83 | 18.49 ± 1.11 | 8.26 ± 0.19 | 3.49 ± 1.47 | 8.41 ± 2.68 | 1.37 ± 0.13 | 2.92 ± 0.58 | 93.98 ± 0.06 |
| PDO3 | 5.51 ± 0.21 | 3.89 ± 0.19 | 0.67 ± 0.13 | 1.13 ± 0.01 | 2.45 ± 0.05 | 1.20 ± 0.01 | 35.52 ± 0.76 | 16.43 ± 0.07 | 7.71 ± 0.58 | 2.65 ± 0.27 | 6.89 ± 0.57 | 1.95 ± 1.25 | 4.42 ± 3.44 | 90.99 ± 1.96 |
| PDO4 | 1.93 ± 0.22 | 2.80 ± 0.56 | 0.19 ± 0.08 | 0.57 ± 0.02 | 0.60 ± 0.06 | Nd | 26.43 ± 1.57 | 20.40 ± 0.13 | 4.60 ± 0.11 | 2.33 ± 0.78 | 3.78 ± 1.26 | 1.55 ± 0.03 | 0.90 ± 0.06 | 66.09 ± 3.17 |
- a) Sample codes are reported in Table 1. Values are expressed as mean ± SD. Nd, not detected; HTyr, hydroxytyrosol; Tyr, tyrosol; VA, vanillic acid; V, vanillin; p-CA, p-coumaric acid; 3,4-DHPEA-AC, hydroxytyrosol acetate; 3,4-DHPEA-EDA, decarboxymethyl oleuropein aglycone; HPin, hydroxypinoresinol; p-HPEA-EDA, decarboxymethyl ligstroside aglycone; Pin, pinoresinol; AcPin, acetoxypinoresinol; Lut, lutein; 3,4-DHPEA-EA, oleuropein aglycone; Api, apigenin; p-HPEA-EA, ligstroside aglycone.
Concerning phenol composition, p-HPEA-EDA + pinoresinol (absent in Carolea) 3,4-DHPEA-EDA + HPin, acetoxypinoresinol (phenol prevalent in Carolea at a level of about 50%), 3,4-DHPEA-EA, and p-HPEA-EA were the most abundant compounds, whereas hydroxytyrosol and tyrosol were detected in very low amounts since analysis was performed within 3 months from oils production. In fact, it is well known that these simple phenols, characterized by potent antioxidant activity, are released during oil storage from complex phenols as secoiridoids by hydrolytic mechanisms 37-39. The great variability found in phenol compounds for monovarietal oils could be ascribed to the different kinetics of hydrolysis occurring by endogenous β-glucosidases activity during crushing and malaxation of olive fruits, and by successive hydrolytic mechanisms in oils that lead to release of simple phenols from complex phenols as secoiridoids 37-39. PDO samples showed a lower variability in phenol composition. This result could be explained considering the cultivars blend adopted in the oils production, as indicated in the official production protocol 20, 21 that reduces the contribution of single cultivars in the final product.
Correlation between antioxidant activity and chemical parameters
On order to evaluate the chemicals able to affect antioxidant activity of oils, the Pearson statistic correlation between AAC and all parameters analyzed was performed (Table 4). Results highlighted a positive correlation between AAC and TPC, p-coumarate (p-CA) and p-HPEA-EA, and a negative correlation with PV. The positive linear correlation between AAC and TPC (r = 0.507) was in agreement with results reported in literature for Spanish and Italian oils 30, 40. However, the lower correlation value obtained in the present work could be explained considering that antioxidant activity depends not only on the phenolic concentration, but also on the specific chemical structure of each phenolic compounds, as demonstrated by Rice-Evans and Miller 41 who indicated a hierarchy for antioxidant activity and reduction potential of phenols. Concerning p-CA, the significant contribution (r = 0.378) of this phenol to the total antioxidant activity was already observed by Owen et al. 42 and Gorinstein et al. 43 in studies on phenolic compounds of olive oil and apple, respectively. Contribute of p-HPEA-EA to antioxidant activity (r = 0.364) was also observed by Baldioli et al. 44 in virgin olive oil. Finally, the negative correlation found between AAC and PV (r = − 0.504) can be ascribed to the low level of oxidation that characterizes fresh virgin olive oil with high AAC. The determination of TPC with Folin–Ciocalteu reagent, although affected by several interfering molecules, represents the most widely used and universal accepted method for phenolics quantification in numerous vegetable food matrixes. A linear regression analysis using AAC as dependent variable and TPC, PV, p-CA, and p-HPEA-EA as independent variables was performed (Table 5). Since a meaningful correlation was found between AAC and TPC and PV, whereas lower for p-CA and p-HPEA-EA, the regression analysis was performed in three steps introducing in following order TPC, TPC, and PV, and TPC, PV, p-CA, and p-HPEA-EA in the regression model. Results highlighted that TPC accounted for the 26% of total variance of AAC. The introduction in the model of PV increased the level to 32%, whereas p-CA and p-HPEA-EA introduction did not significantly improve the model and, consequently, the latter were excluded. The model showed a good fitting with real data as highlighted by the value of Durbin–Watson index that could be considered acceptable (1.374).
| Acidity | PV | K232 | K270 | TPC | HTyr | Tyr | VA | V | p-CA | 3,4-DHPEA-AC | 3,4-DHPEA-EDA + HPin | p-HPEA-EDA + Pin | AcPin | Lut | 3,4-DHPEA-EA | Api | p-HPEA-EA | Total phenols (HPLC) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson r | −0.105 | −0.504a) | −0.146 | 0.099 | 0.507a) | −0.017 | −0.128 | −0.170 | −0.196 | 0.378a) | −0.141 | 0.077 | 0.060 | −0.005 | 0.181 | 0.214 | 0.226 | 0.364a) | 0.233 |
| Sig. (2-tailed) | 0.435 | 0.000 | 0.280 | 0.465 | 0.000 | 0.902 | 0.342 | 0.207 | 0.145 | 0.004 | 0.294 | 0.569 | 0.656 | 0.973 | 0.177 | 0.110 | 0.091 | 0.005 | 0.081 |
- PV, peroxide value; TPC, total phenolic content; HTyr, hydroxytyrosol; Tyr, tyrosol; VA, vanillic acid; V, vanillin; p-CA, p-coumaric acid; 3,4-DHPEA-AC, hydroxytyrosol acetate; 3,4-DHPEA-EDA, decarboxymethyl oleuropein aglycone; HPin, hydroxypinoresinol; p-HPEA-EDA, decarboxymethyl ligstroside aglycone; Pin, pinoresinol; AcPin, acetoxypinoresinol; Lut, lutein; 3,4-DHPEA-EA, oleuropein aglycone; Api, apigenin; p-HPEA-EA, ligstroside aglycone.
- a) Significative correlation.
| Model | R | R2 | Adjusted R2 | Std. error of the estimate | Change statistics | Durbin–Watson | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2-change | F-change | df1 | df2 | Sig. F change | ||||||
| 1 | 0.507a) | 0.257 | 0.244 | 76.65030 | 0.257 | 19.049 | 1 | 55 | 0.000 | |
| 2 | 0.562b) | 0.316 | 0.290 | 74.25196 | 0.058 | 4.610 | 1 | 54 | 0.036 | |
| 3 | 0.579c) | 0.335 | 0.284 | 74.56683 | 0.020 | 0.772 | 2 | 52 | 0.467 | 1.374 |
- a) Predictors: (constant), TPC.
- b) Predictors: (constant), TPC and PV.
- c) Predictors: (constant), TPC, PV, p-CA, and p-HPEA-EA.
Conclusions
The results obtained in the present investigation highlighted a strong variability of TPC and phenolic profiles in oils collected from industrial oil mills located in the Collina di Brindisi PDO geographical area. This variability was more pronounced in monovarietal oils. Apart from variety, ripeness degree of olives was the variable having the greatest impact on phenolics, followed by the extraction system and place of growing. The blend necessary to produce oil in accordance with the PDO production rules, which require the use of at least 70% of Ogliarola cultivar, decreased the differences in content and phenolic profile of monovarietal oils. The antioxidant activity was found to be positively correlated with TPC, p-coumarate, and 3,4-DHPEA-EA, and negatively correlated with PV.
The authors have declared no conflict of interest.




