An efficient reaction protocol for the ruthenium-catalysed epoxidation of methyl oleate
Abstract
The transition metal catalysed epoxidation of methyl oleate 1 by hydrogen peroxide was investigated using Ru(acac)3/dipicolinic acid as catalyst. Under optimised reaction conditions, the epoxidised methyl oleate 2 was obtained with a quantitative yield in short reaction time and under mild reaction conditions.
Practical applications: Epoxidised fatty acids and their derivatives are produced by the Prilezhaev reaction and used in various applications in the chemical industry. Due to the known drawbacks of epoxidation with peroxy acids, such as hazardous handling of peracids in large quantities or the decrease of epoxide selectivity due to the formation of undesired by-products in the acidic medium, the epoxidation of fatty acid derivatives using more convenient oxidants is still a subject of research interest. Herein, we present a simple procedure for the transition metal catalysed epoxidation of methyl oleate 1 by hydrogen peroxide in quantitative yields, and under mild reaction conditions, as a potential alternative for the production of epoxidised fatty acid derivatives.
Introduction
The oxidation of the carbon–carbon double bond of unsaturated fatty acids and their derivatives is an important method in oleochemistry. The industrial processes are the oxidative cleavage of oleic acid by ozonolysis, and the epoxidation of unsaturated plant oils and fatty acid derivatives. Epoxidised plant oils and fatty acid derivatives are used in various applications in the chemical industry. For example, they can be used as a starting material for epoxy resins, as reactive diluents for paints and as intermediates for polyurethane-polyol production. The major application for epoxidised oils is their use as plasticisers and stabilisers in plastics, such as polyvinyl chloride (PVC) 1, 2.
The most widely used method for converting alkenes to epoxides, as well as for the production of epoxidised fatty acids and their derivatives, is the Prilezhaev reaction: this utilises peroxy acids as an oxidant either preformed or in situ prepared by hydrogen peroxide and the corresponding acid 1, 3. Because of the known disadvantage of this process, such as hazardous handling of peracids in large quantities or the decrease of epoxide selectivity due to the formation of undesired by-products in the acidic medium, the epoxidation of fatty acids derivatives is still a subject of research interest 4.
Therefore, numerous methods of catalytic epoxidation with more convenient oxidants have been investigated. The catalytic epoxidation of fatty acids and their derivatives is described using several transition metals such as ruthenium 5, 6, rhenium, tungsten, molybdenum 7, titanium and cobalt by different oxidants such as hydrogen peroxide, alkyl hydroperoxides or oxygen. Also, chemoenzymatic 8, 9 and organocatalytic epoxidation are both described. A review of the oxidation of fatty acids and their derivatives was recently given by Köckritz and Martin 4. Based on the Halcon process for the production of propylene oxide, Ucciani and co-workers 10 described the epoxidation of various fatty acid methyl esters in high yields and selectivities by tert-butyl hydroperoxide or cumene hydroperoxide, with the commercially available heterogeneous catalyst MoO3-Al2O3. Although this process appears to be an attractive method, and oxygen can also be used as a primary oxidant, it is not yet realised for the epoxidation of fatty acid derivatives at a large scale in chemical industry: this is due to the formation of large amounts of tert-butyl alcohol or styrene as by-products 1.
In this context, the transition-metal catalysed epoxidation of fatty acids and their derivatives by hydrogen peroxide is a suitable method for the preparation of epoxides, since hydrogen peroxide is a cheap and also an environmentally friendly oxidant, because water is the only by-product. The aim of our investigations was the development of a practicable catalytic method for the epoxidation of fatty acids derivatives by hydrogen peroxide.
Materials and methods
Materials
Methyl oleate (97%+) was prepared by transesterification of high oleic sunflower oil (Emery olecochemicals) with methanol using catalytic amounts of sulphuric acid. Ru(acac)3 (Degussa), Ru(PPh3)3Cl2 (Acros Organics, 98%), RuCl3 anhydrous (Acros Organics, 99.9%), RuO2 (Acros Organics), [RuCp(CH3CN)3][PF6] (Sigma-Aldrich), [RuCl2(bipy)2] (Alfa Aesar), dipicolinic acid (Acros Organics, 99%), 2,6-pyridinedimethanol (Acros Organics, 98%), picolinic acid-N-oxide (Sigma-Aldrich, 97%), N-methyliminodiacetic acid (Acros Organics, 99%), hydrogen peroxide (Acros Organics, 35%), hydrogen peroxide (Acros Organics, 60%) and solvents (Acros Organics) were used without further purification.
Analytical equipment and methods
The product samples were analysed by GC (Hewlett-Packard HP 6890 with a HP-5 capillary column, 30 m × 0.25 mm × 0.25 µm) using a FID in connection with an auto sampler. Conversion and yields were determined with n-pentadecane as internal standard. GC-MS chromatograms were recorded using a Hewlett-Packard 5973 (HP-5 capillary column, 30 m × 0.25 mm × 0.25 µm), with an ionisation energy of 70 eV.
Experimental procedure
In a typical experiment, 1.48 g (5.0 mmol) methyl oleate 1, 10.0 mg (0.025 mmol) Ru(acac)3 and 83.6 mg (0.50 mmol) dipicolinic acid were dissolved in 20 mL acetonitrile under sonication. After the direct addition of 1.60 g (16.5 mmol) hydrogen peroxide (35% aq), the mixture was stirred magnetically at RT for 3 h. Conversion and yield analyses were performed by GC. The epoxidised methyl oleate 2 was isolated by using flash chromatography on silica-gel with cyclohexene/ethyl acetate (4:1) as eluent, and subsequently characterised by 1H NMR and 13C NMR 11.
Results and discussion
The transition-metal catalysed epoxidation of fatty acid derivatives by hydrogen peroxide was investigated. Methyl oleate 1 was chosen as the model substrate (Fig. 1). The epoxidised methyl oleate 2 is a sustainable and valuable intermediate for the synthesis of environmentally friendly lubricants, e.g., for the 9,10-hydroxyisobutoxystearic acid methyl ester (HISM) 12, and it can also be used for the production of polyurethanes 13.
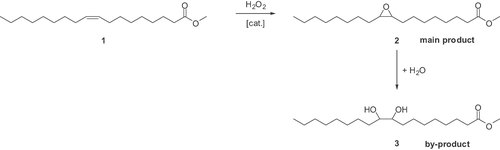
Epoxidation of methyl oleate.
In our investigations of the catalytic oxidation of 1 we found that the catalyst system RuCl3/dipicolinic acid, which is already described as a convenient catalyst for the epoxidation of alkenes by Beller and co-workers 14, is also a suitable catalyst for the selective epoxidation of methyl oleate 1. With the reaction conditions shown in Table 1, the epoxide 2 was obtained with a high yield amounting to 88% (entry 6). Initially, the H2O2 was added slowly by a syringe pump, which made the reaction somewhat unfavourable. During our investigations, the corresponding by-product 9,10-dihydroxystearic acid methyl ester 3 was only formed in insignificant amounts via hydrolysis of 2. This showed that under the reaction conditions no noteworthy subsequent cleavage of the epoxide ring takes place. Also with Ru(PPh3)3Cl2 and Ru(acac)3 high yields up to 90% were obtained (entries 5 and 7). Variation of the ligand shows that only pyridin-2,6-dimethanol gave yields comparable to dipicolinic acid (entry 8). Further ligands were tested but, with the exception of N-methyliminodiacetic acid and picolinic acid N-oxide, no mentionable formation of the epoxide 2 was achieved (entries 9 and 10). Without Ru(acac)3 or dipicolinic acid, formation of epoxide 2 was not observed (entries 1 and 11). In agreement with Beller and co-workers 14, the results showed clearly that both a ruthenium precursor and dipicolinic acid as ligand are required for the effective epoxidation of the C–C double bond.
| Entry | Precursor | Ligand | Conversion 1 (%) | Yield 2 (%) |
|---|---|---|---|---|
| 1 | – | Dipicolinic acid | 2 | 0 |
| 2 | RuO2 | Dipicolinic acid | 4 | 0 |
| 3 | [RuCl2(bipy)2] | Dipicolinic acid | 14 | 6 |
| 4 | [RuCp(CH3CN)3][PF6] | Dipicolinic acid | 99 | 70 |
| 5 | Ru(PPh3)3Cl2 | Dipicolinic acid | 97 | 88 |
| 6 | RuCl3 | Dipicolinic acid | 100 | 88 |
| 7 | Ru(acac)3 | Dipicolinic acid | 100 | 90 |
| 8 | Ru(acac)3 | 2,6-Pyridinedimethanol | 89 | 82 |
| 9 | Ru(acac)3 | N-Methyliminodiacetic acid | 55 | 39 |
| 10 | Ru(acac)3 | Picolinic acid N-oxide | 48 | 40 |
| 11 | Ru(acac)3 | – | 5 | 0 |
- a) Reaction conditions: 5 mmol 1, 0.005 (1 mol%) mmol Ru, 0.050 mmol (10 mol%) ligand, 20 mL tert-butyl alcohol, 25°C, 750 rpm, 12 h slow addition of 16.5 mmol (3.3 eq.) 35% H2O2 in 4 mL tert-butyl alcohol, total reaction time: 16 h.
Due to the in situ formation of the supposed active catalyst Ru(dipicolinic acid)2 (Fig. 2) formed by dipicolinic acid and Ru(acac)3, the Ru/dipicolinic acid ratio was optimised 15. As expected, the variation of the Ru/dipicolinic acid ratio shows a significant influence on both yield and selectivity (Table 2). High yields of the epoxide 2 were observed by increasing the Ru/dipicolinic acid ratio up to 1:20, where a maximum yield of 95% was obtained (entry 5). With a Ru/dipicolinic acid ratio equal to or less than 1:5, the epoxidation occurs in lower yields and at notably minor selectivity (entries 1–3). The results clearly show that for the selective epoxidation of 1 a considerable excess of ligand dipicolinic acid is required. A further increase of the ligand concentration greater than Ru/dipicolinic acid = 1:20 did not result in higher yields of the epoxide 2 (entry 6).
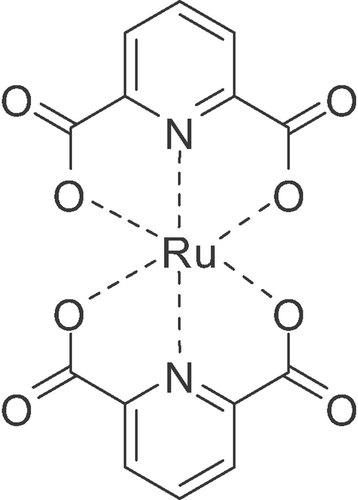
Supposed active catalyst Ru(dipicolinic acid)2.
| Entry | Ratio Ru/dipicolinic acid | Conversion 1 (%) | Yield 2 (%) |
|---|---|---|---|
| 1 | 1:1 | 32 | 17 |
| 2 | 1:2 | 63 | 50 |
| 3 | 1:5 | 91 | 76 |
| 4 | 1:10 | 100 | 90 |
| 5 | 1:20 | 100 | 95 |
| 6 | 1:30 | 100 | 88 |
- a) Reaction conditions: 5 mmol 1, 0.005 (1 mol%) mmol Ru(acac)3, dipicolinic acid, 20 mL tert-butyl alcohol, 25°C, 750 rpm, 12 h slow addition of 16.5 mmol (3.3 eq.) 35% H2O2 in 4 mL tert-butyl alcohol, total reaction time: 16 h.
As summarised in Table 3 the epoxidation of 1 can already be performed with a low catalyst concentration of between 0.1 and 1.0 mol% Ru(acac)3, with yields higher than 94%. A nearly quantitative conversion of 1 and a maximum yield of 98% of epoxide 2 were achieved with 0.5 mol% Ru(acac)3 (entry 4). Even with a very low catalyst concentration of 0.05 mol% Ru(acac)3 the selectivity of the epoxide 2 were still high, but the yield of 2 decreased significantly (entry 1). A high turn over number (TON) of 960 was observed with a catalyst concentration of 0.10 mol% Ru(acac)3 (entry 2).
| Entry | c Ru(acac)3 (mol%) | Conversion 1 (%) | Yield 2 (%) | TON |
|---|---|---|---|---|
| 1 | 0.05 | 40 | 39 | 780 |
| 2 | 0.10 | 95 | 94 | 960 |
| 3 | 0.25 | 96 | 94 | 364 |
| 4 | 0.50 | 99 | 98 | 198 |
| 5 | 1.00 | 100 | 95 | 95 |
- a) Reaction conditions: 5 mmol 1, Ru(acac)3, dipicolinic acid, Ru: dipicolinic acid = 1:20, 20 mL tert-butyl alcohol, 25°C, 750 rpm, 12 h slow addition of 16.5 mmol (3.3 eq.) 35% H2O2 in 4 mL tert-butyl alcohol, total reaction time: 16 h.
Since we were able to perform the epoxidation of 1 with high yields, we tried to make the experimental procedure more simple and economical. As far as this point in the study, the H2O2 had been diluted in tert-butyl alcohol (t-BuOH) and the mixture was slowly added by a syringe pump over a period of 12 h to avoid the non-productive decomposition of H2O2. The priority objective was to shorten the reaction time and to relinquish the unfavourable addition of H2O2 by a syringe pump. Therefore, we investigated the influence of the dosing time for adding the H2O2/t-BuOH solution into the reaction mixture. We increased the flow rate and added the H2O2/t-BuOH mixture over a shortened period. The total reaction time was kept steady at 16 h (Table 4).
| Dosing time H2O2 (min) | Conversion 1 (%) | Yield 2 (%) |
|---|---|---|
| 720 | 99 | 98 |
| 164 | 97 | 93 |
| 80 | 94 | 90 |
| 45 | 97 | 93 |
| Direct addition | 93 | 92 |
- a) Reaction conditions: 5 mmol 1, 0.5 mol% Ru(acac)3, 10 mol% dipicolinic acid, 20 mL tert-butyl alcohol, 25°C, 750 rpm, addition of 16.5 mmol (3.3 eq.) 35% H2O2 in 4 mL tert-butyl alcohol, total reaction time:16 h.
Surprisingly, we found that the flow rate for the addition of the H2O2 does not have a significant influence on the yield of the epoxide 2 and the selectivity of the reaction. Even though the H2O2 was added over a short period of between 45 and 164 min by the syringe pump, the epoxide 2 was achieved in yields higher than 90%. Also, when the H2O2 is completely added at the beginning of the reaction, the epoxide 2 was obtained with a yield of 92%. The results showed that the slow addition of H2O2 by syringe pump is not essential to perform the epoxidation of 1 with high yields, and that the use of the syringe pump is not required. This represents a feasible simplification of the reaction procedure.
However, the slightly lower yield is only acceptable if the long reaction time of 16 h can be shortened by completely adding the H2O2 at the beginning of the reaction. For this purpose, a conversion-time plot of the epoxidation of 1 was performed under the aforementioned reaction conditions. Samples were taken over a period of 6 h and analysed by GC. The resulting time course is presented in Fig. 3. Approximately 64% of 1 was converted within 20 min. The yield of the epoxidation product 2 was further increased within 3 h and amounted to 92%. The by-product 3 was only formed in negligible amounts during the whole period. Thus, the epoxidation proceeds very quickly and the reaction could be reduced from 16 to 3 h. Presumably a 100% conversion of 1 is not achieved because the hydrogen peroxide is depleted after 3 h.
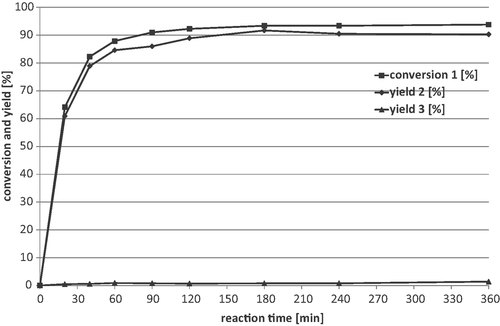
Progress of epoxidation of 1a. a Reaction conditions: 15 mmol 1, 0.5 mol% Ru(acac)3, 10 mol% dipicolinic acid, 49.5 mmol (3.3 eq.) 35% H2O2, 60 mL tert-butyl alcohol, 25°C, 750 rpm, total reaction time: 6 h.
In further investigations, the epoxidation of 1 was performed with different amounts of H2O2 (Fig. 4). A minimum of 3.3 equivalents of H2O2 in relation to methyl oleate 1 was required to achieve yields of the epoxide 2 higher than 92%. It is important to note that with more than 3.3 equivalents of H2O2, only negligible higher yields of the epoxide 2 were formed. With two or less equivalents of H2O2 only yields up to 70% of 2 were obtained.
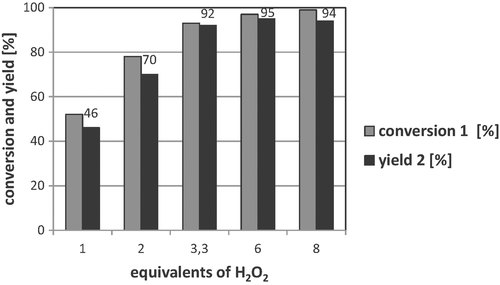
Variation of H2O2-equivalentsa. a Reaction conditions: 5 mmol 1, 0.5 mol% Ru(acac)3, 10 mol% dipicolinic acid, H2O2 (35%), 20 mL tert-butyl alcohol, 25°C, 750 rpm, 3 h.
Given that the epoxidation of the non-polar starting compound 1 is performed with the polar aqueous oxidant H2O2, a suitable solvent for dissolving both the non-polar starting compound 1 and the polar aqueous oxidant H2O2 is required to form a homogeneous reaction solution, and to avoid the application of a phase-transfer catalyst. Therefore tert-butyl alcohol (t-BuOH, entry 2) has been used in the aforementioned investigations to date, and in our variation, mainly medium-polar to polar solvents were used (Table 5). The variation of the solvent showed a significant influence on both yield and selectivity of the reaction. The epoxidation of 1 could be performed in the solvents acetonitrile and 1,4-dioxane with similar high yields and selectivities as in tert-butyl alcohol (entries 1–3). The use of acetonitrile as solvent for the epoxidation of 1 with Ru(acac)3/dipicolinic acid is especially notable. Even using our simplification of the reaction procedure by adding the H2O2 completely at the beginning of the reaction time, and the choice of a shorter reaction time of 3 h, a nearly quantitative yield of epoxide 2 was achieved with acetonitrile as solvent. Primary alcohols, such as methanol and amyl alcohol are not suitable solvents for the epoxidation of 1 because α-alkoxy-hydroxy compounds are formed as side products.
| Entry | Solvent | Conversion 1 (%) | Yield 2 (%) |
|---|---|---|---|
| 1 | Acetonitrile | 99 | 99 |
| 2 | Tert-butyl alcohol | 93 | 92 |
| 3 | 1,4-Dioxane | 87 | 83 |
| 4 | Dimethylformamide | 73 | 73 |
| 5 | Acetone | 96 | 78 |
| 6 | Tert-amyl alcohol | 89 | 75 |
| 7 | Propylene carbonate | 89 | 65 |
| 8 | Methanol | 93 | 76 |
| 9 | Amyl alcohol | 76 | 55 |
| 10 | Tetrahydrofurane | 28 | 22 |
- a) Reaction conditions: 5 mmol 1, 0.5 mol% Ru(acac)3, 10 mol% dipicolinic acid, 16.5 mmol (3.3 eq.) H2O2 (35%), 20 mL solvent, 25°C, 750 rpm, 3 h.
Investigations of the substrate concentration show that, under the chosen reaction conditions, a solvent volume of 20 mL (concentration of 1 in acetonitrile: 0.25 mol/L) is necessary to obtain high yields of 2. With a higher concentration of 1 (0.50 and 1.00 mol/L), both the yield and the selectivity of epoxide 2 decreased. The reaction could also be performed with 60% aqueous H2O2 instead of 35% aqueous H2O2. Under reaction conditions given in Table 5 and with acetonitrile as solvent, epoxide 2 was obtained in 99% yield with both oxidants.
Conclusions
In conclusion, we have shown that Ru(acac)3/dipicolinic acid is an efficient catalyst system for the selective epoxidation of methyl oleate 1 by 35% H2O2. At the beginning of the investigations the addition of the oxidant H2O2 was performed by a syringe pump in order to avoid the decomposition of the H2O2, which is somewhat inconvenient but also preferred in previous publications. In contrast to this, the systematic investigation of the reaction conditions showed that the H2O2 can be directly added to the reaction mixture and resulted in a simple reaction procedure for the preparation of the epoxide 2. A major benefit of the catalyst system is that no significant opening of the epoxide ring by water to the diol 3 occurs, although the reaction is performed with aqueous hydrogen peroxide. Under the optimised reaction conditions, the epoxidation of 1 could be performed with a quantitative yield of the epoxide 2 within a short reaction time of 3 h, under mild reaction conditions.
Acknowledgements
This work was financially supported by the German Federal Ministry of Food, Agriculture and Consumer Protection (represented by the Fachagentur Nachwachsende Rohstoffe) and Emery Oleochemicals. The authors thank Dr. Alfred Westfechtel for helpful discussions.
The authors have declared no conflict of interest.




