Synthesis and properties of novel alkyl sulfate gemini surfactants
Abstract
Four anionic gemini surfactants of the sulfate type C12CnC12, where n is the spacer chain length (n = 3, 4, 6, and 10) were synthesized. The structures of these surfactants were confirmed by FT-IR, 1H NMR, ESI mass spectra (ESI-MS), and elemental analysis. The surface-active properties of these compounds were investigated by means of surface tension, electrical conductivity, and fluorescence measurements. Premicellar aggregations were found for the four gemini surfactants, as revealed by the conductivity measurement. The formation of premicellar aggregates may account for the discrepancy between the critical micelle concentration (cmc) obtained by the surface tension and conductivity measurement. The cmc values of these gemini surfactants were much lower than that of sodium dodecylsulfate (SDS) and decreased monotonously with the increase of spacer chain length from 3 to 10. The effect of spacer chain length on the performance properties like foaming, emulsion stability, and lime soap dispersing ability were also studied and discussed.
Practical applications: Alkyl sulfate surfactants are one of the most widely used surfactants. The new alkyl sulfate gemini surfactants synthesized in our study are more surface-active than sodium dodecylsulfate. These gemini surfactants possess low critical micelle concentrations, high emulsion stability, and excellent lime soap dispersing ability. They have potential applications in the fields of cosmetics, detergents, etc.
Abbreviations:
C12CnC12, anionic gemini surfactants synthesized in this article, where n refers to the spacer carbon number of 3, 4, 6, and 10; ESI-MS, ESI mass spectra; LSDR, lime soap dispersing requirement; SDS, sodium dodecylsulfate; cmc, critical micelle concentration; THF, tetrahydrofuran
Introduction
Gemini surfactants are a new generation of surfactants which emerged in the last decades. They are made up of two hydrophobic alkyl chains and two hydrophilic headgroups connected by a spacer at the level of headgroup or close to the headgroup, thus gemini surfactants can be considered as dimers of conventional surfactants with one hydrophobic tail and one hydrophilic headgroup. Gemini surfactants have been proved to be superior in many aspects compared with conventional surfactants, such as lower critical micelle concentration (cmc), greater efficiency, and effectiveness in surface tension reduction, better water solubility, wetting property, and lime soap dispersing ability, etc 1-4. Due to the unique properties, gemini surfactants have attracted worldwide attention both in academic research and industrial production.
Up to now, the most studied gemini surfactants are cationic geminis. Their surface-active properties and aggregation properties are well investigated. However, there are relatively few investigations concerning anionic gemini surfactants, especially anionic sulfate geminis 5-8. At present, the structure–performance relationship of these surfactants is far from being understood. Alkyl sulfate surfactants are one of the oldest surfactants in the surfactants family. These surfactants often possess excellent wetting, dispersing, emulsifying, and cleaning properties and are widely used in the fields of household and personal products, such as detergents, shampoos, textile auxiliaries, toothpastes, and so on 9. Thus it would be interesting and important to design and synthesize new kinds of alkyl sulfate gemini surfactants and get more information about these compounds.
Based on these considerations, we have synthesized four anionic sulfate gemini surfactants with different spacer chain length in this paper, the structures are listed in Figure 1. These surfactants can be regarded as dimers of sodium dodecylsulfate (SDS). Their surface-active properties were studied by surface tension, electrical conductivity and fluorescence measurements. We have also studied their foaming, emulsifying, and dispersing properties and try to get a better understanding of the influence of spacer chain length on the performance properties of gemini surfactants.
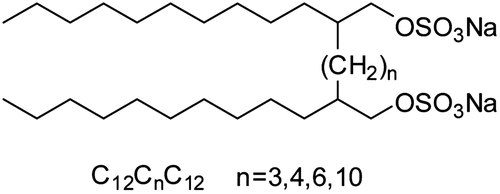
Chemical structure of the gemini surfactants studied in this work.
Materials and methods
Materials and instruments
Diethyl malonate, potassium t-butoxide, 1,3-dibromo propane, 1,4-dibromo butane, 1, 6-dibromo hexane, 1,10-dibromo decane, 1-bromodecane, chlorosulfonic acid were purchased from Sinopharm chemical reagent corporation (Shanghai, China) and used as received. THF (tetrahydrofuran) was refluxed overnight with Na and benzophenone and distilled before use. Chloroform was washed with water and dried with calcium chloride. Pyrene (Alfa Aesar, USA) was 99% pure and recrystallized three times from ethanol. SDS (Alfa Aesar, USA) was 99% pure and used as received. Doubly distilled water was used in all experiments. Other reagents were all commercial available and used as received.
FT-IR spectra were recorded on a model 5700 FT-IR spectrometer (Thermo Nicolet Corporation, USA), and KBr pellets of the samples were used to analysis. 1H NMR spectra were performed on a Mercury VX 300 MHz spectrometer (Varian, USA) with DMSO-d6 as solvent. ESI mass spectra (ESI-MS) were obtained on a P/ACE MDQ CE-MS spectrometer (Beckman Coulter, USA). Elemental analysis was determined on a Vario EL (Elementar, Germany) elemental analyzer. Surface tension was measured by the Wilhelmy plate technique using a QBZY-1 tensiometer (FANGRUI Corporation, Shanghai, China) equipped with a platinum plate. Electrical conductivities of surfactant solutions were measured using an inoLab Cond 730 conductivity meter (WTW, Germany). The fluorescence intensities were measured using an F-4600 FL spectrophotometer (Hitachi, Japan).
Synthesis and characterization
The synthesis of the gemini surfactants used in this study is illustrated in Figures 2 and 3. The synthetic route of 5a-d was based on FitzGerald et al. 10, with some modifications.
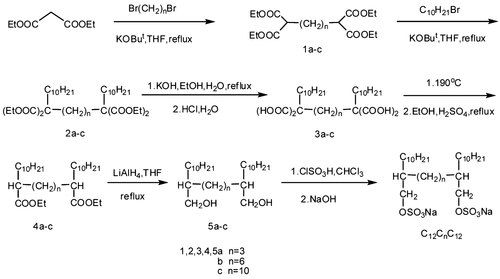
Synthetic route of C12C3C12, C12C6C12, and C12C10C12.
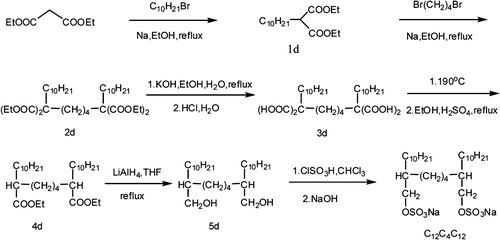
Synthetic route of C12C4C12.
Synthesis of 1a-d
For the synthesis of 1a-c, potassium t-butoxide (0.375 mol) was dissolved in dry THF (400 mL), and diethyl malonate (0.45 mol) was added dropwise to the mixture under stirring, followed by the addition of dibromoalkane (0.15 mol) in a dropwise manner. After the addition of dibromoalkane, the mixture were stirred and heated to reflux, the reaction was continued for 21 h and then cooled to RT, water (1200 mL) was added, the mixture were extracted with 3:1 ether-dichloromethane (600 mL). The organic layer was washed twice with water (2 × 500 mL), dried, and concentrated. Excess diethyl malonate was removed by vacuum distillation; further distillation of the residue under reduced pressure gave 1a and 1b as oils in 38 and 80% yields, respectively. The boiling point of 1c was too high to be purified by vacuum distillation, after the removal of excess diethyl malonate, the crude product was used in the next step without further purification. In the case of 1d, 0.1 mol metallic sodium was dissolved in 150 mL anhydrous alcohol, then 0.12 mol diethyl malonate was added, the mixture was heated to 70°C for 30 min and then cooled to RT, 0.09 mol 1-bromodecane was added and the reaction mixture was continued for 12 h under reflux. The solvent was removed by rotary evaporation, water (100 mL) was added to the residue, followed by the extraction of ether (3 × 50 mL), the organic layer was combined, washed with water (2 × 30 mL), dried, and concentrated. Distillation of the crude product under reduced pressure gave 1d as colorless oil in 82% yield.
Synthesis of 2a-d
For the synthesis of 2a-c, 0.06 mol tetraester (1a-c) was added to a stirred solution of potassium t-butoxide (0.132 mol) in 120 mL dry THF, followed by the addition of 1-bromodecane. The mixture was heated to reflux under stirring and the reaction was continued for 40 h, and then cooled and stirred with 10% hydrochloric acid (15 mL) for 5 min. After evaporation of the solvent under reduced pressure, the residue was extracted with ether (400 mL), the ether layer was washed with water (4 × 200 mL), dried with Na2SO4. Evaporation of the solvent gave 2a-c as pale yellow oils. The crude product was used in the next step without further purification. In the case of 2d, 0.075 mol metallic sodium was dissolved in 150 mL anhydrous alcohol, then 1d (0.07 mol) was added, the mixture was heated to 70°C for 30 min and then cooled to RT, 0.035 mol 1,4-dibromo butane was added and the reaction mixture was continued for 36 h under reflux. The solvent was removed by rotary evaporation, water (70 mL) was added to the residue, followed by the extraction of ether (3 × 50 mL), the organic layer was combined, washed with water (2 × 30 mL), dried with Na2SO4. Evaporation of the solvent gave 2d as pale yellow oil. The crude product was used in the next step without further purification.
Synthesis of 3a-d
0.06 mol 2a-d and 0.72 mol KOH were mixed with 400 mL ethanol, the reaction was stirred and refluxed for 18 h, and then water (300 mL) was added, the resulting solution was stirred for a further 4 h, more water (300 mL) was added, and the pH of the solution was adjusted to 1 by concentrated hydrochloric acid. The mixture was cooled to ice temperature; the precipitate formed was collected by filtration, washed with cold water and dried to constant weight to afford 3a-d as pale yellow solids. The crude product was used in the next step without further purification.
Synthesis of 4a-d
0.04 mol 3a-d was placed in a 500 mL round-bottled flask, a gas-measuring device was connected to the flask. The flask was immerged into an oil bath, which was preheated to 190°C. After the evolution of CO2 gas ceased, the system was cooled to 90°C. Ethanol (270 mL) was added to the flask, followed by the addition of concentrated sulfuric acid (9 mL) in a slow dropwise manner. The reaction was stirred and refluxed for 5 h and then cooled. After evaporation of the solvent, water (300 mL) was added to the residue, followed by the extraction of ether (400 mL), the organic layer was washed with water (4 × 100 mL), dried, and concentrated. Silica gel column chromatography, typically with petroleum ether: ethyl acetate (50:1 v/v), gave compounds 4a-d with yields of 36–52%.
Synthesis of 5a-d
4a-d (0.035 mol) dissolved in dry THF (250 mL) was added dropwise to a stirred suspension of LiAlH4 in dry THF (130 mL) at RT under argon atmosphere. The reaction was stirred at RT for 1 h and then refluxed for a further 8 h, the mixture was then allowed to cool to ice temperature and quenched by the addition of water (25 mL). The reaction was refluxed for a further 1 h, the white precipitate was removed by filtration, and the filtrate was dried and concentrated. Silica gel column chromatography, typically with petroleum ether: ethyl acetate (7:1 v/v), gave compounds 5a-d with yields of 83–92%.
Synthesis of C12CnC12
-
C12C3C12: FT-IR (cm−1, KBr): 2957 (CH stretch in CH3), 2923 (CH stretch in CH2), 2853 (CH stretch in CH3), 1466 (CH bend in CH3), 1248, 1204, 1079, 834 (SO); 1H NMR (300 MHz, DMSO-d6, δ in ppm): 0.86 (6H, t), 1.24 (46H, m), 1.48 (2H, m), 3.58 (4H, d). ESI-MS (negative): m/z 593.6 [MNa]−, 571.5 [M2Na+H]−, 285.6 [M2Na]2−. Elemental analysis for (C27H54Na2O8S2) Calculated (%): C, 52.57; H, 8.82. Found (%): C, 52.09; H 8.66.
-
C12C4C12: FT-IR (cm−1, KBr): 2924 (CH stretch in CH2), 2854 (CH stretch in CH3), 1466 (CH bend in CH3), 1221, 1074, 829 (SO); 1H NMR (300 MHz, DMSO-d6, δ in ppm): 0.86 (6H, t), 1.24 (48H, m), 1.48 (2H, m), 3.58 (4H, d). ESI-MS (negative): m/z 607.6 [MNa]−, 292.6 [M2Na]2−. Elemental analysis for (C28H56Na2O8S2) Calculated (%): C, 53.31; H, 8.95. Found (%): C, 52.65; H 9.20.
-
C12C6C12: FT-IR (cm−1, KBr): 2924 (CH stretch in CH2), 2854 (CH stretch in CH3), 1466 (CH bend in CH3), 1249, 1218, 1073, 827 (SO); 1H NMR (300 MHz, DMSO-d6, δ in ppm): 0.81 (6H, t), 1.20 (52H, m), 1.43 (2H, m), 3.54 (4H, d). ESI-MS (negative): m/z 635.8 [MNa]−, 613.6 [M2Na+H]−, 306.6 [M2Na]2−. Elemental analysis for (C30H60Na2O8S2) Calculated (%): C, 54.69; H 9.18. Found (%): C, 53.13; H 9.32.
-
C12C10C12: FT-IR (cm−1, KBr): 2924 (CH stretch in CH2), 2853 (CH stretch in CH3), 1466 (CH bend in CH3), 1218, 1070, 824 (SO); 1H NMR (300 MHz, DMSO-d6, δ in ppm): 0.86 (6H, t), 1.24 (60H, m), 1.46 (2H, m), 3.56 (4H, d). ESI-MS (negative): m/z 691.7 [MNa]−, 334.7 [M2Na]2−. Elemental analysis for (C34H68Na2O8S2) Calculated (%): C, 57.11; H, 9.59. Found (%): C, 56.44; H, 9.92.
Surface tension
The surface tensions of the surfactant solutions were measured by the Wilhelmy plate method. Sets of measurement was taken at 10 min intervals until the change in surface tension were <0.2 mN/m. Surface tension of doubly distilled water was measured regularly to check the validity of the data. All the measurements were performed at 25°C using a constant-temperature bath.
Electrical conductivity
A concentrated stock solution of the surfactant (about 10 times the cmc) was added progressively to 50 mL water in small portions (500 µL) using a microliter syringe. After each addition, the solution was thoroughly mixed and the electrical conductivity was recorded. The temperature of surfactant solutions was maintained at 25 ± 0.1°C during the experiment.
Steady-state fluorescence measurement
The fluorescence spectra of pyrene solubilized in surfactant solutions were recorded between 350 and 450 nm at an exciting wavelength of 335 nm. The slit widths of excitation and emission were both 2.5 nm. The concentration of pyrene was 10−3 mmol/L in each surfactant solution. Surfactant solutions containing fluorescence probe were ultrasonicated 2 h before measurement. The measurements were done at RT (ca. 25°C).
Foaming
Foaming experiments were carried out according to a procedure described elsewhere 11, 12. A 100 mL stoppered cylinder was used for the measurements, the cylinder was thermostated at 30°C using a constant-temperature bath. Ten milliliters of 0.1% solution of each surfactant in the stoppered cylinder was vigorously shaken for 10 s, foam volume was recorded immediately. The time required for the collapse of the foam to half of its initial height (half-life) was also recorded and used for the evaluation of foam stability. The experiments were repeated three times and the mean values were given.
Emulsion stability
Emulsion stabilities of the surfactants were studied by mixing 40 mL of 0.1% surfactant solutions and 40 mL mineral oil in a 250 mL Erlenmeyer flask. The mixture was vigorously shaken up and down five times and stayed for 1 min. Emulsion was obtained by repeatedly shaking the mixture and stayed at 1 min intervals for five times. The emulsion formed was immediately poured into a 100 mL glass cylinder. The time of separation of 10 mL water from the emulsion was recorded for the evaluation of emulsion stability. The recorded data were the mean values of three experiments.
Lime soap dispersing requirement (LSDR)
Lime soap dispersing requirement (LSDR) of the surfactants were carried out according to the procedure of Borghetty and Bergman 13. The minimum amount of dispersing agent required to disperse completely calcium soap formed by 100 g sodium oleate in 333 ppm hard water were used to evaluate the dispersing power. LSDR were expressed as percentage by dividing the weight of surfactant by weight of the sodium oleate.
Results and discussion
Critical micelle concentration
Two methods are used to evaluate the cmc values of the gemini surfactants synthesized, the surface tension measurement, and the conductivity measurement. Figure 4 shows plots of the surface tension (γ) of synthesized gemini surfactants as a function of log surfactant molar concentration (logC), cmc values are taken as the break point of the γ-logC curve. Although carefully purified, the trace impurities in C12C10C12 seem more surface-active than C12C10C12. The γ-logC curve of C12C10C12 shows no clear break point and the cmc value can't be obtained, thus the data of C12C10C12 are discarded. The obtained cmc and surface tension at the cmc (γcmc) of C12C3C12, C12C4C12, and C12C6C12 are described in Table 1. The cmc values are also obtained by the conductivity measurement. As a representative, the specific conductivities K of C12C4C12 solutions as a function of concentration are plotted in Figure 6, cmc value is taken as the break point in the conductivity versus surfactant concentration plot. The cmc values obtained by the conductivity measurement are also collected in Table 1. It is noteworthy the cmc values obtained by the surface tension method are considerably lower than these obtained by the conductivity method. Similar results have been observed by Pinazo et al. 14 for a kind of cationic gemini surfactants derived from arginine, and later by Biesen and Bottaro 8 for a kind of alkyl sulfate gemini surfactant with a hydrophilic poly(ethylene oxide) spacer. They noticed that the cmc values obtained by conductivity and fluorescence measurement are close to each other but considerably higher than these obtained by surface tension measurement. These authors gave the explanation that small, non-globular aggregates are formed in the submicellar concentration range. To address this issue, we examined the conductivity data of these gemini surfactants and SDS. Figures 5 and 6 show the change of specific conductivity K with concentration C and of molar conductivity Λ = (K − K0)/C with C0.5 (K0 is the specific conductivity of pure water) for SDS and C12C4C12, respectively. The plots for SDS show a normal behavior, however, this is not the case of C12C4C12. As shown in Figure 6, there is a slight upward curvature at concentrations below the cmc in the K versus C plot, and a pronounced maximum in the Λ versus C0.5 plot in the submicellar concentration range. This is a typical behavior of premicellar aggregation 15. The Λ versus C0.5 plot for C12C3C12, C12C6C12, and C12C10C12 also show a maximum, which indicates the formation of premicellar aggregations.
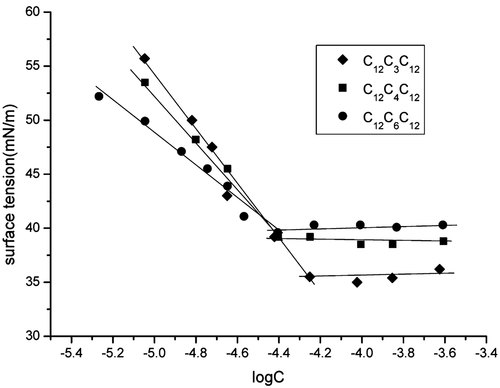
Change of surface tension with the logarithm of surfactant concentration for C12C3C12, C12C4C12, and C12C6C12 at 25°C.
| Surfactants | cmca) (mM) | cmcb) (mM) | γcmc (mN/m) | 1010 Γmax (mol/cm2) | Amin (nm2) |
|---|---|---|---|---|---|
| C12C3C12 | 0.056 | 0.39 | 35.5 | 1.50 | 1.11 |
| C12C4C12 | 0.041 | 0.37 | 39.1 | 1.35 | 1.24 |
| C12C6C12 | 0.038 | 0.30 | 40.2 | 0.88 | 1.89 |
| C12C10C12 | — | 0.18 | — | — | — |
| SDS | 8.0 |
- a) By surface tension measurement.
- b) By conductivity measurement.
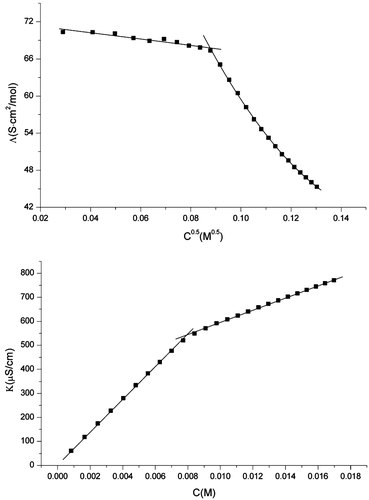
Change of specific conductivity K with concentration C and of the molar conductivity Λ with C0.5 for SDS at 25°C.
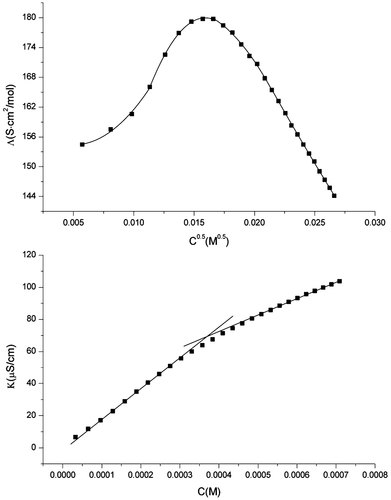
Change of specific conductivity K with concentration C and of the molar conductivity Λ with C0.5 for C12C4C12 at 25°C.
To give a better understanding of the micellization progress, we performed the fluorescence measurement using pyrene as a fluorescence probe. Pyrene is widely used as a fluorescence probe in the study of surfactant micellization. The intensity ratio of the first (I1) and third (I3) peaks in the pyrene fluorescence spectrum is a sensitive parameter for the evaluation of polarity around the probe 16. When the surfactant concentration is low, pyrene is solubilized in water and senses a high polarity. I1/I3 decreases rapidly when the surfactant concentration increases, which indicate the formation of micelles. For a surfactant with low cmc, the concentration corresponding to the mid-decrease of the I1/I3 in the curve was used to determine the cmc, as proposed by Zana et al. 17. The variation of I1/I3 versus surfactant concentration is plotted in Figure 7. Usually, the curve shows a rapid decrease when micelles are formed and tends to level off at high concentration ranges of surfactants. However, in the case of C12CnC12, the I1/I3 ratio shows a gradual decrease over a wide concentration range, and the cmc could not be accurately designated. Despite of this, we roughly estimate the cmc values of C12CnC12, they are 0.25, 0.27, 0.41, and 0.29 mM for C12C3C12, C12C4C12, C12C6C12, and C12C10C12, respectively. The cmc values obtained by conductivity and fluorescence measurement are close to each other but differ much from these obtained by surface tension measurement. Furthermore, at the concentration of cmc obtained by the surface tension method, the I1/I3 ratio for C12C3C12, C12C4C12, and C12C6C12 close to that of water, suggesting no strongly hydrophobic environment formed. Combining the results of surface tension, conductivity, and fluorescence measurements, it is reasonable to speculate that the discrepancy between the cmc values obtained by the surface tension and conductivity measurements might be caused by the formation of premicellar aggregates. In these cases, the conventional cmc values refer to these obtained by electrical conductivity method.
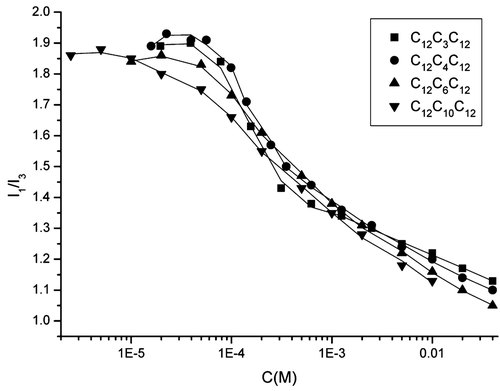
Change of I1/I3 with concentration of C12CnC12.
As is shown in Table 1, the cmc of gemini surfactants synthesized are one order of magnitude lower than the corresponding monomeric surfactant SDS. The gemini surfactants investigated show excellent micelle-forming properties as compared to SDS. It can also be seem from Table 1 that the cmc decreases monotonously with the increase of spacer length from 3 to 10. The dependence of cmc with spacer length for the gemini surfactants investigated in our research agrees with previously reported results for other anionic gemini surfactants 18, 19. When the spacer is short enough, the spacer is stretched due to the electrical repulsion of the anionic headgroups. Along with the increase of spacer length, the spacer may progressively bend to the micelle hydrophobic core, thus the free energy of micellization decreases and result in a lower cmc.
The surface excess concentration (Γmax), minimum surface area per molecule (Amin)
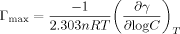
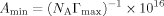
Foaming
Foaming properties of surfactants investigated are studied at a concentration of 0.1% for gemini surfactants and 0.5% for SDS. The concentrations of surfactants investigated are all above their corresponding cmc. The measured data are shown in Table 2. It is evident that the four gemini surfactants show lower foamability compared to SDS, especially, C12C10C12 shows very low foamability, only a very small volume of foam generated from the experiment, so it is meaningless to discuss its foam stability. However, high foam stabilities are observed for the other three gemini surfactants. The very long half-lives indicate extremely stable foam formed by the gemini surfactants. Indeed densely packed small-bubble foam is observed for the gemini surfactants studied.
| Surfactants | Foam properties | Emulsion stability (s) | LSDR | |
|---|---|---|---|---|
| Initial foam height (mL) | Half-life (min) | |||
| C12C3C12 | 40 | >180 | 105 | 12 |
| C12C4C12 | 27 | >180 | 125 | 13 |
| C12C6C12 | 17 | >180 | 125 | 13 |
| C12C10C12 | <5 | — | 133 | 12 |
| SDS | 110 | 44 | 58 | 30 |
Emulsion stability
The emulsions formed by mixing mineral oil and surfactant solutions are studied. The concentrations of gemini surfactants and SDS are 0.1 and 0.5%, respectively. Emulsion stability is evaluated by the time of 10 mL water separated from the emulsion. The measured data are depicted in Table 2. It is found that the emulsion stability of gemini surfactants is better than that of SDS. However, emulsion stability shows no obvious difference for the gemini surfactants with different spacer length.
Lime soap dispersing requirement
Lime soap dispersing requirement of a lime soap dispersing agent reflects the ability to disperse insoluble lime soaps. The lower LSDR value means a better lime soap dispersing ability. Table 2 lists the LSDR values of gemini surfactants, the LSDR value of SDS is also listed as a reference. The LSDR of gemini surfactants is about 13, which is much lower than that of SDS. This means the lime soap dispersing ability is greatly improved when going from SDS to its dimers. However, the four gemini surfactants give almost identical LSDR values, irrespective of their spacer length. This indicates the spacer has little influence on the lime soap dispersing ability of the gemini surfactants investigated.
The authors have declared no conflict of interest.




