Effect of extra virgin olive oil on glycaemia in healthy young subjects
Abstract
In this approach we studied the glycaemia levels in 20 healthy young volunteers (26 ± 2 years), before and after a 30-day intake of 50 mL of extra virgin olive oil (EVOO). We selected an oil rich in phenolic compounds (523 mg/L) with a high content of secoiridoidic derivatives (over 94.5%). The findings from our study reveal a significant decrease of glycaemia from 89.6 ± 6.8 to 82.7 ± 10.3 mg/dL (p<0.05), related to a long term daily intake of the study EVOO, as the only added fat. A significant increment of the HDL cholesterol, from 68.7 ± 11.5 to 75.2 ± 4.9 mg/dL, was also highlighted. Total cholesterol, LDL, VLDL, triglycerides, and blood pressure did not show significant variation after the 30-day consumption of this EVOO. So far, few articles have described the influence of EVOO consumption, on plasma glucose levels in humans. This effect is observed in a group of healthy young humans. Moreover, we confirm that the level of free hydroxytyrosol (OH-Tyr) in plasma increased up to fourfold (p<0.05) after the 30-day intake of this EVOO. In addition, the excretion in urine of the main metabolite of OH-Tyr, homovanillic acid (HVA), significantly increased.
Abbreviations:
3,4-DHPE-EA, oleuropein aglycone; 3,4-DHPE-EDA, deacetoxy oleuropein aglycone; COMT, catechol-O-methyl-transferase; EVOO, extra virgin olive oil; HVA, homovanillic acid; HVAlc, homovanillic alcohol; OH-Tyr, hydroxytyrosol; Tyr, tyrosol
Introduction
The evaluation of the beneficial effects of polyphenols from extra virgin olive oil (EVOO) has been the scope of many studies during these last years. Olive oil consumption has shown to reduce oxidative damage and cardiovascular risk in European populations 1, 2. Some authors report to have found increased HDL cholesterol 3-5, increased antioxidant capacity 6-8, and gene expression from antioxidant enzymes 6, 9 in intervention periods with participants consuming daily EVOO.
In addition, previous papers also reported decreased levels of blood pressure 5, 10, platelet aggregation 5, 11, 12, homocysteine 12, triglycerides 6, 13, 14, LDL cholesterol, and total cholesterol levels 5, 6, 13, 14, in intervention studies with EVOO. Oxidized LDL 1, 3, 4, 7, 15, 16, oxidative stress in several tissues 4-6, 11, 17-19 and DNA oxidative damage 20 have also shown to be reduced mainly due to the effect of polyphenols from EVOO.
Phenolic compounds seem also to have an effect on insulin sensitivity and glycaemia levels. In fact, Iwai 21 highlighted that a polyphenolic extract from a brown alga reduced glucose levels in plasma of diabetic mice. Similarly, Awwadi et al. 22 evidenced that a red wine polyphenol extract induced a reduction in food intake and glycaemia in both healthy and diabetic rats. In addition, a reduction of the glucose levels in diabetic rats fed with cocoa extract has been proved 19. Few papers have evaluated the effect of consuming dark chocolate 23, tea 24, 25, and olive oil 26, 27 on the decrease of postprandial plasma glucose and insulin resistance in healthy and diabetic human subjects.
Recently Hamdem et al. 28, demonstrated for the first time, that olive mill waste polyphenols and especially hydroxytyrosol (OH-Tyr), were efficient in inhibiting hyperglycaemia and oxidative stress induced by diabetes in rats. A recent paper evidenced the capacity of oleuropein aglycone (3,4-DHPE-EA) in the prevention of citotoxic amyloid aggregation of human amylin, a hallmark of Type II diabetes 29. The health effects of polyphenols depend on their respective intakes and bioavailability, which can vary greatly 8, 15, 30, 31 mainly depending on the oil dose, short or long term ingestion, and time of collection of biological samples (plasma and urine). It is well known that EVOO phenols, especially OH-Tyr, are adsorbed in humans in a dose-dependent manner 30, 32-34. Moreover, most of the findings related to studies on single dose, suggested an extensive first-pass intestinal and hepatic metabolism of the ingested OH-Tyr so determining a high amount of conjugated forms, mainly glucuronide conjugates 32, 33.
The aim of this work is to verify the glycaemia levels in 20 healthy young volunteers, before and after a 30-day intake of 50 mL of an EVOO. Moreover, total cholesterol, LDL, VLDL, and triglyceride levels did not change before and after the intervention period. In addition, plasmatic and urinary phenolic concentrations were also measured. The selected oil was rich in phenolic compounds and had the peculiarity to contain a high amount of secoiridoidic derivatives of oleuropein, compounds containing the linked forms of OH-Tyr.
Materials and methods
Chemicals
Tyrosol (Tyr), catechol, homovanillic acid (HVA) were from Sigma–Aldrich (St. Louis, MO, USA) and hydroxytyrosol was purchased from Cayman Chemical (SPI-BIO, Europe). All reagents used for HPLC analysis were of analytical grade. The SPE cartridges were Oasis HLB 3cc, from Waters (Milford, MA).
Study subjects
Twenty healthy young volunteers participated in the study. Ethical approval for the study was obtained from the Bioethical Research Committee of the University of Granada (Spain). All procedures involving subjects complied with the Declaration of Helsinki, as revised in 2000. All subjects were informed and signed a written consent prior taking part in the study. Volunteers were selected on the basis of the following criteria: 20–30 years, body mass index between 18 and 25 kg/m2, non-smoking, normolipidaemic, normoglucaemic, absence of hypertension, diabetes, cardiovascular, or any other chronic diseases. None of the volunteers took any antioxidant, dietary supplements, or any medication. Anthropometric, dietetic, and biochemical characteristics of the healthy young volunteers at baseline (day 0) and after the period of study (day 30) are described in Table 1.
| Baseline | EVOO | p value | |
|---|---|---|---|
| Anthropometric parameters | |||
| Number | 20 | 20 | |
| Sex (M/F) | 8/12 | 8/12 | |
| Age (years) | 26 ± 2 | 26 ± 2 | |
| Weight (kg) | 63 ± 2a | 62.5 ± 2a | 0.4341 |
| Height (cm) | 170 ± 6a | 170 ± 6a | 0.9958 |
| BMI (kg/m2) | 21.8 ± 2.2a | 21.7 ± 2.1a | 0.8839 |
| Waist/hip ratio | 0.7 ± 0.1a | 0.7 ± 0.1a | 0.9499 |
| Systolic blood pressure (mm Hg) | 110 ± 8a | 110 ± 8a | 0.9062 |
| Diastolic blood pressure (mm Hg) | 65 ± 6a | 65 ± 6a | 0.9166 |
| Pulse rate (frequency/min) | 68 ± 4a | 68 ± 4a | 0.8752 |
| Dietetic intake | |||
| Energy intake (Kcal) | 2546 ± 1006a | 2581 ± 872a | 0.907 |
| Protein (% total energy) | 16.3 ± 1.8a | 15.8 ± 2.1a | 0.4239 |
| Carbohydrates (% total energy) | 48.7 ± 4.7a | 49.1 ± 5.9a | 0.8138 |
| Fats (% total energy) | 31.6 ± 4.4a | 32.1 ± 4.7a | 0.7303 |
| Saturated fatty acids (%) | 7.5 ± 2a | 6.5 ± 1.7a | 0.0966 |
| Monounsaturated fatty acids (%) | 17.2 ± 4.4a | 19.6 ± 3.8a | 0.0787 |
| Polyunsaturated fatty acids (%) | 6.1 ± 2.9a | 4.8 ± 2.6a | 0.1438 |
| Vitamin C (mg) | 151.2 ± 68.4a | 143.7 ± 72.5a | 0.7383 |
| Vitamin E (mg) | 11.9 ± 4.8a | 14.2 ± 5.2a | 0.1543 |
| Vitamin A (µg) | 2243.7 ± 986.5a | 2368.4 ± 1302.6a | 0.7352 |
| Added fats (g/d) | |||
| EVOO | 22.3 | 64.8 | |
| Seed oils | 15.2 | 0 | |
| Butter | 9.8 | 0 | |
| Margarine | 15 | 0 | |
| Animal fat | 0 | 0 | |
| Biochemical parameters | |||
| Total cholesterol (mg/dL) | 184 ± 6.6a | 180.5 ± 6.5a | 0.4486 |
| LDL cholesterol (mg/dL) | 102.4 ± 7.5a | 99.1 ± 4.9a | 0.4486 |
| HDL cholesterol (mg/dL) | 68.7 ± 11.5a | 75.2 ± 4.9b | 0.024 |
| VLDL cholesterol (mg/dL) | 13 ± 4.5a | 12.6 ± 4.8a | 0.7872 |
| Triglycerides (mg/dL) | 65.5 ± 4.7a | 64.3 ± 5.2a | 0.4486 |
| Glucose (mg/dL) | 89.6 ± 6.8a | 82.7 ± 10.3b | 0.0168 |
- a,bDifferent letters in the same column indicate significantly different values (p<0.05).
Experimental study design
The present research consisted of a long term trial with healthy young subjects, to study the influence of daily ingestion of an EVOO rich in phenolic compounds, as the only added fat. Each participant was involved in the study for 6 weeks. The trial was divided in two periods, a first period of 2 weeks and a intervention period of 4 weeks, starting at day 0 (baseline). Prior to study, they were interviewed and informed with detail about the procedures of the study. Throughout the 6 weeks of the study, all participants were instructed to continue their normal life style, without modifying their usual physical activities. We did not incorporate a wash-out period because our purpose was to examine, in healthy young people, the effects of replacing usual added fat ingestion by the study olive oil.
All through the first 2-week period, no foodstuff was limited. Participants reported their dietary habits and they were recommended to not modifying them. Moreover, they were asked to consume their habitual fats and oils in this period. Data from this 2-week period were collected at baseline. Volunteers were individually advised about how to proceed and achieve diet compilation. Dietetic characteristics were assessed by a semi-quantitative food frequency questionnaire (FFQ) of 136 items validated in Spain 35. This validated questionnaire has recently confirmed the reproducibility of a previously validated FFQ 36. The questionnaire gives nine options for frequency of consumption of the different foods and food groups. The portion size usually used for each item was indicated (slice, spoon, glass…) so that the volunteers reported how often they had consumed each unit 35. In addition, participants also compile a 7-day weighed food diary of the consumed diet. All questionnaires were checked by a nutritionist and average nutrient intakes were calculated and converted into nutrients using the software Dietsource 3.0 (Novartis, Spain). Anthropometric and biochemical parameters were measured.
Subsequently, throughout the 4-week period of intervention, subjects did not modify neither qualitative nor quantitative characteristics of their diet, with the exception of the consumption of oil. The nutritionist gave personalized explanations to each volunteer especially about the use of oil. They were asked to use the study oil for cooking and seasoning food, instead of all fats and oils usually consumed. Volunteers were instructed to consume daily 50 mL of the study oil in raw form, in addition to the study oil necessary for cooking. The study oil was supplied at baseline to each volunteer. They were provided 50 mL units to cover daily raw oil ingestion. Study oil was also provided in 2 L containers in enough quantity to be sufficient to use it for cooking. The dose of raw oil was about equally distributed among meals (breakfast: 25 mL; lunch: 25 mL). The oil consumed in meals was estimated by reported serving size. Apart from the fat substitution, subjects were instructed to stay on their habitual diet. This pattern was followed for 30 days. During the intervention period the participants also fulfilled the validated FFQ 35 and the 7-day weighed food diary, so that average intakes of nutrients were evaluated. Anthropometric and biochemical parameters were also measured at day 30 (Table 1).
Subjects were request to be fasting for 12 h before the blood analysis. Blood samples were drawn at baseline (morning of day 0) and at the end of the intervention period (morning of day 30). Standard protocol for blood collection recommended by professional organisations was used (Procedure of blood extraction, Spanish Association of Medical Biopathology). Venipuncture was performed by a trained phlebotomist. Blood for the hematology and biochemical determination was collected in a vacutainer tube containing anticoagulant (BD Vacutainer with lithium heparin; Becton Dickinson, Madrid, Spain). The freshly withdrawn venous blood was gently inverted eight times immediately after collection to prevent clotting. Collection tubes were correctly labeled and a requisition form accompanied each sample submitted to the laboratory. Blood for polyphenols measurements was collected into another vacutainer tube containing sodium citrate (BD Vacutainer). This tube was also gently inverted eight times and after labeled. All blood samples were stored in containers with ice and kept in the dark. Special care was taken to avoid exposure to air, light, and ambient temperature. The samples were processed within the hour after extraction. Plasma was separated by centrifugation of the blood samples at 1500 rpm for 20 min at 18–25°C. Plasma samples were aliquoted in cryovials adequately labeled and stored at −80°C until analyzed by HPLC. The first spot morning urine samples were also collected at baseline (morning of day 0) and at the end of the intervention period (morning of day 30). Total cholesterol, VLDL, and triglycerides were measured using enzymatic tests. LDL cholesterol levels were calculated by the Friedewald equation. HDL cholesterol was measured by using the heparin–manganese precipitation method and fasting plasma glucose was assayed by the glucose oxidase method 37.
The study oil was an EVOO from olive variety Picual (from Andalusia, Spain), pressed at 18–22°C in a two-phase centrifugation system and characterized for its phenolic content (Table 2).
| Phenolic Compounds | Concentration [mg/L]a) |
|---|---|
| OH-Tyr | 1.68 ± 0.05 |
| Tyr | 3.33 ± 0.07 |
| 3,4-DHPE-EA | 72.97 ± 4.42 |
| 3,4-DHPE-EDA | 88.67 ± 8.86 |
| Other secoiridoids | 339.8 ± 3.9 |
| Luteolin | 1.48 ± 0.09 |
| Apigenin | 0.61 ± 0.2 |
| Pinoresinol | 13.81 ± 1.77 |
| Total phenols | 522.35 |
- a) Mean ± SD of three determinations.
HPLC-DAD-MS analysis of phenolic compounds of extra virgin olive oil
Phenolic compounds, extracted according to Oliveras-López et al. 38 were analyzed by an Agilent 1100 liquid chromatograph equipped with a 1100 autosampler, column heater module, binary pump and DAD; the MS detector was a HP 1100 MSD API-electrospray, all from Agilent Tech. (Palo Alto, AC, USA). The Luna RP18 (Phenomenex-Torrance CA) column, 250 mm × 4.6mm (5 µm), and the LiChrosorb RP18 (Merk Hibar) column, 250 mm × 4.6 mm (5 µm) were used to quantify all the phenolics in the oil. The qualitative–quantitative determination was performed as reported in a previous paper 38.
Recovery of phenolic compounds from plasma and urine
Plasma samples were purified by the use of reverse phase cartridges 6, 39. The catechol (0.5 µg as IS) was added to 1 mL of plasma and the sample was applied on the cartridge previously conditioned with 1 mL of methanol (MeOH) and equilibrated with 1 mL of H2O. The washing step was with 3 mL of H2O and the recovery of the phenols was in 2 mL of MeOH, added with 10 µL of ascorbic acid. Then it was dried and redissolved in 200 µL of MeOH and injected 50 µL.
Urine samples were purified by the use of reverse phase cartridges after a little modification of the method applied for plasma samples 39. The urine samples (4 mL) were freeze dried, then, redissolved in 2 mL of distilled water and centrifuged. The cartridge was previously conditioned with 1 mL of MeOH and 8 mL of acid water (pH 3.2 by HCOOH). The catechol (1 µg as IS) was added to 2 mL of sample and was then applied on the cartridge. The washing step was performed with 8 mL of acid water and the recovery of the phenols was in 2 mL of MeOH, added with 10 µL of ascorbic acid. Then it was dried and dissolved in 1 mL of acid water and injected 25 µL.
The HPLC was carried out in the instrument described before with a Synergy max column (150 × 4 mm2; 5 µm, Phenomenex); the solvents were acid water (pH 3.2 by HCOOH) (A) and acetonitrile (B). The following gradient elution was applied for the plasma samples: from A 97% to A 90% in 25 min; 2.5 min to A 85%, then 7.5 min to A 0% and a plateau for 5 min. Total time of analysis 40 min, equilibration time 10 min, oven temperature 30°C; flow rate 0.4 mL/min. The operative conditions of the MS detector were capillary voltage from 3000 to 3500 V, working in negative and positive ionization, with a variable fragmentor (80–200 V).
Quantitative determination
The amount of HVA was evaluated in the urine samples, while in the plasma only the OH-tyrosol was determined; by the use of the two pure standards the calibration curves were constructed by the use of HPLC/API/ES using the signals correspondent to the pseudo molecular ions in negative ionization (fragmentor 80 V) of 181 m/z for the HVA and 153 m/z for the OH-Tyr.
Statistical analysis
Values were expressed as mean values with their standard errors. The obtained data were analyzed through the statistical package, SPSS 17.0. (SPSS, Inc.). After checking the normalcy of the variables and verifying the inexistence of significant gender-based differences, the t-test and non-parametric tests were performed, as applicable, in order to evaluate the statistical differences between the subjects characteristics. Non-normal variables were analyzed by the non-parametric Wilcoxon test for two related samples. Differences were considered significant for p<0.05.
Results and discussion
The purpose of this study was to examine, in healthy young people, the effects of replacing usual added fat ingestion by the study EVOO, rich in secoiridoidic constituents. It was focused to evaluate glycaemia levels in 20 healthy young volunteers, before and after a 30-day intake of 50 mL of an EVOO. Moreover, total cholesterol, LDL, VLDL, and triglyceride levels did not change before and after the intervention period. All participants completed the 6-week study. Diet with the study oil was well tolerated by all subjects. Their anthropometric, dietetic and biochemical characteristics at baseline (day 0) and after the intake of the study EVOO (day 30) are summarized in Table 1. Compared to the baseline diet, the intervention diet did not modify energy intake. At baseline the average daily energy intake was 2546 ± 1006 kcal, with a 31.6% energy intake coming from fats. The evaluation of dietary recalls showed no significant changes in average energy intake (2581 ± 872 kcal) neither in the percentage of fats (32.1%) during the 4-week period of EVOO consumption.
Diet analysis showed increased monounsaturated acids after the period of EVOO intake, while saturated and polyunsaturated acids decreased. However, these changes were not significant. In fact, increase of vitamin E was small, with no significant value. Diet cholesterol, fiber, and alcohol intake remained with no modifications. There were no changes in other nutrients, compared to baseline.
Table 1 shows that there were no changes of body weight, blood pressure, total cholesterol, LDL, and triglycerides during the trial. The intervention period was associated with a significant reduction of glycaemia and a significant increase of HDL levels, compared to baseline. So far, few articles have described the influence of EVOO consumption, on plasma glucose levels in humans. The findings from our study pointed out a significant decrease of glycaemia (Fig. 1) from 89.6 ± 6.8 mg/dL to 82.7 ± 10.3 mg/dL (p<0.05), not related to an acute single dose as previously described 26, 27, but after a long term daily intake of 50 mL of an EVOO rich in secoiridoidic constituents. It can be assessed that this effect is observed for the first time in a group of healthy young humans.
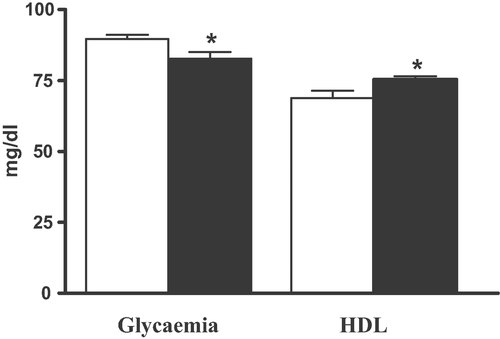
Glycaemia (mg/dL) and HDL cholesterol levels (mg/dL) in humans before (white) and after (black) the intake of the study EVOO for 30 days. *p<0.05.
It has been addressed the role of dietary fatty acids in the development of insulin resistance and the association of increased monounsaturated acids with lower fasting plasma glucose concentrations 40. Furthermore, clinical trials have demonstrated that administration of antioxidant vitamins improved insulin sensitivity or were associated with fasting plasma glucose 41. However, in our study the intake of fatty acids and antioxidant vitamins did not modified significantly, as shown in Table 1. This could be due to the fact that the study subjects habitually consumed olive oil as the most frequently consumed added fat. It is not unusual to hypothesize a correlation between the glucose plasma level and the phenolic intake from different sources. Several authors have highlighted beneficial effects after assumption of dark chocolate 23 and green tea 25 on the glycaemia regulation. A recent work put in evidence hypoglycaemic effects of phenolic extracts and purified hydroxytyrosol in rats 28.
Our results (Fig. 1) highlighted also a significant increment of the HDL cholesterol, from 68.7 ± 11.5 mg/dL at baseline to 75.2 ± 4.9 mg/dL (p<0.05) after the EVOO intake. At the same time, the total cholesterol, LDL, VLDL, triglycerides, and blood pressure did not show significant variation after the daily consumption of this EVOO for 1 month. These findings agree with previous results that pointed out an analogous increment of HDL on subjects of 25–47 years 3. Several papers reported the ability of the olive oil and EVOO to increase the HDL in humans 3-5 and to reduce total or LDL cholesterol levels 4, 5, 13. Our study has the limitation of the small number of participants. In fact, the lack of significant changes in some biochemical parameters could be due to insufficient statistical power. However, most of the previous reports were targeted to adults selected within a wide age range and often taking in account only a single dose assumption 31. On the opposite, few works 3, 33 have been focused to evaluate the effects in humans after a long term consumption of EVOO well characterized in terms of phenolic compounds.
On the other hand, it could be argued that the reduction of glycaemia or the increase of HDL was due to the fat replace in addition to other changes of diet during the intervention period. However, volunteer compliance was verified at each visit and was evaluated by the dietary recalls. Subjects did not modify their diet, with the exception of the consumption of the study oil, so that food intake (apart from added fat replace) before and during the test period was the same. Since most of the recent studies have highlighted a strong correlation between the beneficial properties of EVOO and its phenolic concentration 3, 4, 7, 10, 16, 20, in this research we selected an oil rich in phenolic compounds, with high amounts of secoiridoids, mainly oleuropein derivatives (Table 2). It has been recently pointed out the capacity of 3,4-DHPE-EA to prevent amyloid aggregation of human amylin, a hallmark of Type II diabetes 29. It is well known that secoiridoids are the natural source of OH-Tyr and Tyr in the EVOOs 42. In this perspective, an evaluation of the OH-Tyr concentration in plasma and of HVA in urine before and after the period of EVOO consumption was also done. As shown in Fig. 2, the level of free OH-Tyr in plasma increased up to fourfold (p<0.05) after the 30-day intake of this EVOO.
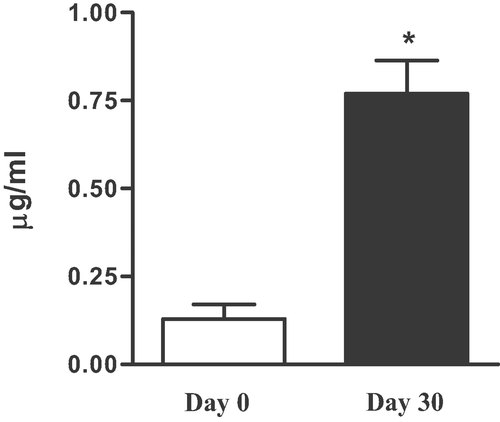
Plasma hydroxytyrosol levels (µg/mL) in humans before (white) and after (black) the intake of the study EVOO for 30 days. *p<0.05.
Homovanillic acid, the main metabolite of OH-Tyr, was detected in urine. In addition, measurements showed that the excretion in urine of this compound significantly increased (Fig. 3). Plasma and urine changes after the intake of the study oil supported good compliance. Our results are in agreement with previous papers indicating an increase of urinary excretion of HVA in subjects consuming EVOO after a single dose ingestion 20, 30, 32, and an increment of OH-Tyr and Tyr also after long term supplementation 3. The high excretion of OH-Tyr and its metabolites in urine is related to the dose supplied of free OH-Tyr 30, but also to the linked forms to the secoiridoidic nucleo 43, indicating an in vivo hydrolysis of secoiridoids from EVOO. In humans, the catechol-O-methyl-transferase (COMT) system is responsible for the metabolic conversion of OH-Tyr to homovanillic alcohol (HVAlc), that can be oxidized to HVA, the final metabolite of OH-Tyr in urine 30. Caruso et al. 32, after a single dose ingestion of EVOO, highlighted a concentration of HVA seven-times higher than OH-Tyr amount in urine, showing HVA is the major metabolite found in urine. This could explain the detection of only HVA in our urine samples (Fig. 4).
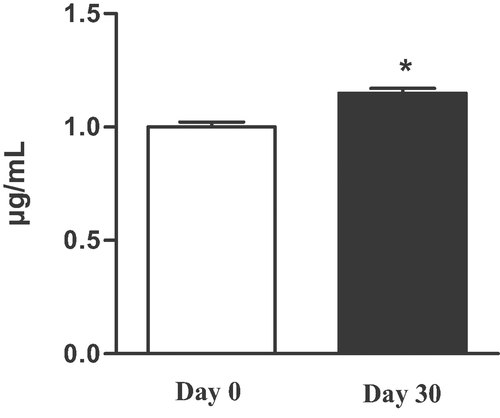
Urine HVA levels (µg/mL) in humans before and after the intake of the study EVOO for 30 days. *p<0.05.
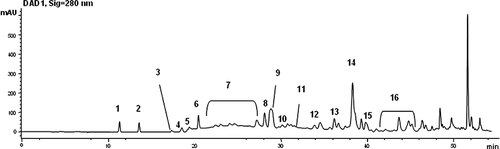
HPLC–DAD chromatograms at 280 nm of the Picual EVOO phenolic extract. Identified compounds: 1, OH-Tyr; 2, Tyr; 3, Tyr derivatives; 4–7, 11, 13, 15, 16, Secoiridoids derivatives; 8, impure luteolin and derivatives; 9, pinoresinol; 10, unknown; 12, impure apigenin and derivatives; 14, 3,4-DHPE-EA or oleochantal.
In conclusion, the findings from our study reveal a significant decrease of glycaemia related to a long term daily intake of an EVOO rich in phenolic compounds, mainly secoiridoidic derivatives, as the only added fat. Therefore, there is evidence that the replacement of added fats by EVOO can play a prevention role even in healthy young people.
Acknowledgements
The authors are grateful to Ente Cassa di Risparmio di Firenze for supplying part of the instrumentation used for this research.
The authors have declared no conflict of interest.




