Protective effect of dietary perilla oil on allergic inflammation in asthmatic mice
Abstract
Perilla oil (PER) is rich in α-linolenic acid (n-3 fatty acid). To unravel the effects of dietary PER on allergic asthmatic inflammation, three kinds of dietary oil, including PER, corn oil (COR), and perilla compound oil (50% PER and 50% COR), were used for replacing the oil in an AIN76 feed consumed by ovalbumin (OVA)-sensitized and challenged mice continuously for 5 wk. T-helper type 1 lymphocyte (Th1)/T-helper type 2 lymphocyte (Th2) and pro-/anti-inflammatory cytokines secreted by the cells from the airway, the lungs, and the spleen of experimental mice were determined by ELISA. The results showed that dietary PER inhibited interleukin (IL)-1β and tumor necrosis factor (TNF)-α secretions by lipopolysaccharide (LPS)-stimulated lung cells, as well as interferon (IFN)-γ and IL-6 secretions by LPS-stimulated splenocytes. Perilla compound oil increased the secretion ratio of IFN-γ/IL-5 (Th1/Th2 cytokines) in LPS-stimulated bronchoalveolar lavage fluid cells, but decreased the ratio of IL-6/IL-10 (pro-/anti-inflammatory cytokines) in LPS-stimulated splenocytes. The present study demonstrated that dietary PER and its compound oil protected the airways, the lungs, and the spleen from allergic inflammation in OVA-challenged asthmatic mice, suggesting that an appropriate n-6/n-3 fatty acid ratio at a ratio of 1:1 or less in dietary oil may be beneficial to improve the Th2-skewed allergic asthmatic inflammation.
Practical applications: The present study demonstrated that dietary PER and its compound oil protected the airways, the lungs, and the spleen from allergic inflammation in OVA-challenged asthmatic mice, suggesting that an appropriate n-6/n-3 fatty acid ratio at a ratio of 1:1 or less in dietary oil may be beneficial to improve the Th2-skewed allergic asthmatic inflammation.
Abbreviations:
ALNA, α-linolenic acid; BALF, bronchoalveolar lavage fluid; COR, corn oil; COX, cyclooxygenase; EPA, eicosapentaenoic acid; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; NF, nuclear factor; OVA, ovalbumin; PER, perilla oil; Th1, T-helper type 1 lymphocyte; Th2, T-helper type 2 lymphocyte; TLR, toll like receptor; TNF, tumor necrosis factor
Introduction
Multi-symptom asthma, which is related to nasal blockage, rhinorrhea, and symptoms of chronic rhinosinusitis, is now a profound worldwide public health problem with a prevalence of about 5–10% in different populations 1. Asthma is recognized as an acute or chronic inflammatory disorder of the airways and lungs resulting from the interaction of cells and mediators 2, 3. There are two distinct subtypes of helper T cells, T-helper type 1 (Th1) and type 2 (Th2) lymphocytes, which secrete specific cytokines that modulate with reciprocal inhibition 4, although Th1/Th2 dichotomy still cannot be used to totally understand the complex interactions in the control of immunological homeostasis and both IL-17 expressing (Th17) cells and Foxp3+ regulatory T cells are important in controlling the inflammatory development 5. Th1 cells are characterized by the production of interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α, possibly enhancing inflammation to remove antigens in vivo. In contrast, Th2 cells-secreted cytokines include IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13. Among Th2 cytokines, IL-10 is also produced by regulatory T cells, macrophages, and some B cells during the late phase of inflammation. IL-10 is a cytokine synthesis inhibitory factor and is a potent suppressant of macrophage functions. Macrophages are called pro-inflammatory cells, therefore IL-10, in some cases, is called an anti-inflammatory cytokine for its inhibitory activities of cytokine synthesis and macrophage functions 6. There is a good balance between Th1 and Th2 immune responses in healthy person. However, an imbalance between the Th1 and Th2 immune responses is associated with many diseases. Th1-skewed immune responses are generally pro-inflammatory and may result in autoimmune and chronic inflammatory diseases, on the other hand, Th2-skewed immune responses may cause asthma and allergy. Recently, asthma was found to have at least two distinct molecular phenotypes according to degree of Th2 inflammation: Th2-driven asthma and non-Th2-driven asthma 7. Th2-driven inflammation, mediated by IL-4, IL-5, and IL-13, defines major subphenotypes of asthma, however non-Th2-driven asthma that still cannot be adequately explained by current models represents a significant proportion of patients and responds poorly to current therapies 7. Therefore, dietary or drug therapies targeting Th2 lymphocytes and maintaining the Th1/Th2 balance in vivo may be effective in Th2-driven asthma patients 8.
Recently, dietary n-3 PUFA have been suggested to treat asthma although only few significant effects were found 9. Among the sources of the n-3 fatty acids, perilla oil (PER), which is obtained from the seeds of Perilla frutescenes, consists of about 50–60% α-linolenic acid (ALNA, 18:3n-3) and exhibits high n-3 fatty acid bioavailability 10. It was found that PER plays different physiological roles, including the inhibition of the growth of Staphylococcus aureus and the production of exotoxins in vitro 11, the inhibition of monocyte recruitment to inflamed intestinal mucosa in the senescence accelerated mice (SAM)P1/Yit mouse model 12, as well as the protection of mitochondrial function in the brain in guinea pigs 13. In our previous studies, dietary PER also demonstrated many physiological effects including hypolipidemia and immunomodulation in vivo via decreasing the secretion levels of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) and Th1 cytokines (IFN-γ and IL-2) in bronchoalveolar lavage fluid (BALF) of ovalbumin (OVA)-sensitized and challenged mice, suggesting that dietary PER alleviated inflammation via decreasing the secretion of pro-inflammatory cytokines in BALF 14, 15. However, chronic asthma may cause both local and systemic inflammation. The spleen collects antigen from the blood and is involved in immune responses to blood-borne pathogens, possibly reflecting the systemic inflammation status. To further understand which tissues in asthmatic subjects were affected by dietary PER, this study further investigated the effects of dietary PER on the airways, lungs, and spleen of asthmatic mice.
We hypothesized that dietary PER has anti-inflammatory effects on the airways, lungs, and spleen in asthmatic mice. To unravel the possible protective effects of dietary PER on allergic asthmatic mice, primary cells, respectively, isolated from the airways (BALF), lungs, and spleen of OVA-challenged experimental mice were cultured in the absence or presence of lipopolysaccharide (LPS). The secretion levels of pro-/anti-inflammatory and Th1/Th2 cytokines secreted by these cells were determined. Based on the changes in cytokine secretion profiles, the protective effect of dietary PER on the target tissues in asthmatic mice were clarified.
Materials and methods
Materials
PER rich in ALNA (>60.5%) was purchased from MasterAsia Marketing Co. (Taipei, Taiwan, ROC) as the source of n-3 fatty acid. There are approximate 60.5% ALNA, 14.7% linoleic acid, 16.1% oleic acid, 2.0% stearic acid, and 6.2% palmitic acid in PER according to the manufacturer's profiles. Corn oil (COR), rich in linoleic acid but poor in ALNA (<2%), was purchased from Chang Chi Foodstuff Factory Co. (Taichung, Taiwan, ROC) as a control and the source of n-6 fatty acid. The fatty acid composition in COR consists of 49.3% linoleic acid, 28.5% oleic acid, 2.1% stearic acid, and 12.9% palmitic acid 14, 15. Compound oil (COR-PER) was a mixture containing 50% COR and 50% PER.
Experimental animals grouping and feeds
The feed formula was same as that described by Chang et al. 14, 16. The experimental feed was prepared according to the recommendation of the American Institute of Nutrition AIN-76 that satisfies the nutritional requirement for mouse growth and varied only in lipid composition. The basic composition of each feed, expressed in g/100 g, contained 40 g sucrose, 25 g corn starch, 20 g casein, 5 g fiber, 3.5 g mineral mixture, 1 g vitamin mixture, 0.3 g DL-methionine, 0.2 g choline bitartrate, and 5 g lipid. The components of each feed were thoroughly mixed and stored at −20°C. There were three kinds of lipids (PER, COR, and COR-PER) used in the experiment. The animal use protocol listed below has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC), National Chung Hsing University, Taiwan, ROC. The female BALB/cByJNarl mice (6-wk-old) were obtained from the National Laboratory Animal Center, National Applied Research Laboratories, National Science Council in Taipei, ROC and maintained in the Department of Food Science and Biotechnology at National Chung Hsing University College of Agriculture and Natural Resources in Taichung, Taiwan, ROC. The animal room was kept on a 12-h light and 12-h dark cycle. Constant temperature (25 ± 2°C) and humidity were maintained. The mice were housed and kept on a chow diet (laboratory standard diet, Diet MF 18, Oriental Yeast Co., Ltd., Osaka, Japan) to acclimatize for 2 wk before feeding the experimental diet. After this equilibrium period, mice were randomly divided into four groups (n = 12) varied by oil source and sensitized treatments: non-sensitized control (treated with phosphate-buffered saline; PBS and alum (Al(OH)3), coded as PBS/COR), dietary control (OVA/COR), OVA/COR-PER, and OVA/PER. The initial average body weight of each group showed no significant differences among groups. Each group was fed with the specified experimental diet for 35 consecutive days ad libitum. Mice food intake and body weight were measured twice a week during the study period. All experimental mice survived and there were no significant differences in food intake, body weight, and appearances of mice among groups during the experimental period.
OVA-sensitized and -challenged allergic asthmatic inflammation mouse model
The allergic asthmatic inflammation murine model has been used in our previous studies 14-16. The mice (8-wk-old) were sensitized and challenged to induce allergic airway inflammation. The mouse allergic airway inflammation model was manipulated as described by Lin et al. 17 and slightly modified at aerosolized-OVA concentration and inhalation time to enhance the induction of airway inflammation. In brief, mice were sensitized using an intraperitoneal injection (i.p.) of 0.2 mL alum-precipitated antigen containing 8 µg of OVA (albumin chicken egg grade III, Sigma–Aldrich Co., St. Louis, MO, USA) and 2 mg Al(OH)3 (Sigma–Aldrich Co.) to induce primary immunity after supply of the specified experimental diets for 1 wk. Two booster injections of this alum–OVA mixture were given 7 and 14 days later, respectively. Non-sensitized control mice received alum–PBS (137 mM NaCl (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 2.7 mM KCl (Sigma–Aldrich Co.), 8.1 mM Na2HPO4 (Sigma–Aldrich Co., Steinhein, Germany), 1.5 mM KH2PO4 (Sigma–Aldrich Co.), pH 7.4, 0.2 µm filtered) only. One week later, the OVA-sensitized mice were challenged using aerosolized OVA at a concentration of 5 mg OVA per milliliter PBS for 60 min, twice a day at 3 days intervals. The aerosolized OVA were produced using an ultrasonic nebulizer (sw918, Shinmed, Taipei, Taiwan). Non-sensitized control mice received only PBS. Two days later, the animals were anesthetized with isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane, Sigma, MO, USA), exsanguinated using retro-orbital venous plexus puncture and immediately euthanized by CO2 inhalation.
Isolation and culture of BALF cells, lung cells, and splenocytes
BALF cells isolation
The BALF collection was modified from the methods described by Ye et al. 18. After the mice were euthanized, the airways and the lungs were immediately lavaged aseptically using a cannula through the trachea with five aliquots of 0.6 mL Hank's balanced salts solution (HBSS), free of ionized calcium and magnesium (HyClone Laboratories Inc., Utah, USA). The BALF was centrifuged at 400 × g for 10 min at 4°C. The BALF cell pellet was collected and resuspended in MEM medium containing 10% fetal bovine serum (FBS, Biological Industries Inc., Kibbutz Beit Haemek, Israel), 4 mM of L-glutamine (Sigma, Steinhein, Germany), 25 mM of HEPES (Biological Industries Inc.), 5 × 10−5 M of 2-mecaptoethanol (Sigma), and 0.5% of Penicillin–Streptomycin Amphotericin B Solution (100×, contained 1000 units/mL penicillin, 10 mg/mL streptomycin, and 0.025 mg/mL amphotericin, Biological Industries Inc.) in MEM-ALPHA medium (Biological Industries Inc.). The total cell count was performed with a hemocytometer using the trypan blue dye exclusion method. Because total cells from some individual mice were insufficient for the experimental cell density, isolated cells from 2 to 4 individual mice in same groups were pooled together to achieve the desired cell density. The cell density was adjusted to 1 × 106 cells/mL in MEM medium. To achieve a fixed cell density, BALF cells from ∼3 individual mice in the same group were pooled. BALF cytocentrifuged preparations were stained with Liu's stain for differential cell counts. Based on standard morphologic criteria, BALF cells were easily identified as macrophages, lymphocytes, or eosinophils 14.
Lung cells isolation
After BALF cell collection, the lung cells were collected aseptically using the methods described in Xie et al. 19 and Maximiano et al. 20 with a slight modification. In brief, the lungs were excised and the blood washed off with 0.9% NaCl sterilized solution. The tissue was then minced finely with scissors and incubated in MEM-ALPHA medium containing 30 µg/mL DNase (DN25, Sigma, Missouri, USA) and 150 units/mL collagenase (C0130, Sigma) for 30 min at 37°C. The lung fragments were filtered through a Cell Strainer (70 µm nylon mesh, Falcon 352350, BD, NJ, USA). Cell suspensions were centrifuged at 400 × g for 7 min at 25°C. The cell pellet was resuspended in MEM medium. The cells were counted with a hemocytometer using the trypan blue dye exclusion method. Because total cells from some individual mice were insufficient for the experimental cell density, isolated cells from two individual mice in same groups were pooled together to achieve the desired cell density. The cell density was adjusted to 1 × 106 cells/mL in MEM medium.
Splenocytes isolation
The splenocytes were prepared by aseptically removing spleens from the experimental BALB/c mice. The spleen was ground with the flat bottom of a syringe piston to homogenize the splenocytes. Splenocytes were centrifuged at 400 ×g for 7 min at 25°C. The cell pellets were resuspended in 10 mL of red blood cell (RBC) lysis buffer (0.017 M Trizma Base (Sigma–Aldrich Co.) and 0.144 M NH4Cl (Sigma–Aldrich Co.) in deionized water, pH 7.2, 0.2 µm filtered) for 3 min and centrifuged at 400 × g for 7 min at 25°C. The cell pellets were washed with HBSS three times. Splenocytes were resuspended in TCM medium that contained 20% of TCM Serum Replacement (Protide Pharmaceuticals Inc., Illinois, USA) and 0.5% of Penicillin–Streptomycin Amphotericin B Solution in RPMI 1640 medium (HyClone Laboratories Inc.). The cells were counted with a hemocytometer using the trypan blue dye exclusion method. Because total cells from some individual mice were insufficient for the experimental cell density, isolated cells from two individual mice in same groups were pooled together to achieve the desired cell density. The cell density was adjusted to 1 × 107 cells/mL in TCM medium. The cellular purity of B and T cells in normal female BALB/c mice was assayed by FACS analysis. The splenocytes consisted of 41.5% B cells and 47.1% T cells, respectively 21.
Cultures of BALF cells, the lung cells, and splenocytes
The isolated BALF cell, the lung cell, and splenocyte suspensions were plated into 48-well plates (Nunc, Thermo Fisher Scientific, IL, USA), and, respectively, incubated with or without LPS (L2654, Sigma). The final LPS concentration in the wells was 5 µg/mL. The plates were incubated at 37°C in a humidified incubator with 5% CO2 and 95% air for 48 h. The cell culture supernatants were collected and stored at −80°C for cytokine assays.
Measurement of cytokine levels in the cell culture supernatants by an ELISA
Cytokine (IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IFN-γ, and TNF-α) levels in the supernatants of cell cultures were determined using sandwich ELISA kits, respectively. The IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IFN-γ, and TNF-α concentrations were assayed according to the cytokine ELISA protocol in the manufacturer's instructions (mouse DuoSet ELISA Development system, DY401E, DY402E, DY404E, DY405E, DY406E, DY410E, DY417E, DY485E, R&D Systems, MN, USA). The sensitivity of these cytokine assays was <15.6 pg/mL.
Statistical analysis
Values were expressed as mean ± SEM. Data of lung cells and splenocytes within OVA-sensitized and -challenged treatments were analyzed using ANOVA, followed by Duncan's multiple range test. Data of lung cells and splenocytes between the dietary control and non-sensitized control were analyzed using ANOVA, followed by unpaired Student's t-test. Data of BALF cells were first analyzed by Kruskal–Wallis (KW) test. If the statistical probability p<0.05 analyzed by the KW test, Mann–Whitney U-test was further used for comparisons between two random groups. Differences among groups were considered statistically significant if p<0.05. Statistical tests were performed using SPSS version 12.0 (SPSS, Inc., Chicago, IL, USA).
Results and discussion
Effects of dietary oils on anti- and pro-inflammatory cytokine levels in the LPS-stimulated lung cell cultures from OVA-challenged mice
To evaluate the anti-inflammatory effects of dietary oils on the lungs in OVA-challenged mice, an endotoxin LPS was administered to the lung cell cultures to induce inflammation. Table 1 shows the levels of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) and an anti-inflammatory cytokine (IL-10) in the LPS-stimulated lung cell cultures from OVA-challenged mice fed different experimental diets through 5 wk. As our expectation, the spontaneous (no LPS stimulation in vitro) cytokine secretion levels by the lung cells were too low to be detectable (data not shown). However, the OVA-sensitization and -challenge markedly (p<0.05) increased the IL-6 secretion from 1.56 ± 0.88 to 4.13 ± 0.82 µg/mL (an increase of 164.7%) by the LPS-stimulated lung cells (Table 1). PER administration in vivo, either PER alone (OVA/PER) or its compound oil containing PER and COR (OVA/COR-PER), decreased the levels of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) and an anti-inflammatory cytokine (IL-10), compared to those of the OVA/COR group. Interestingly, the dietary compound oil significantly (p<0.05) inhibited IL-1β (decreased from 35.6 ± 5.9 to 13.2 ± 2.3 pg/mL, a decrease of 62.9%), TNF-α (decreased from 315 ± 57 to 166 ± 7 pg/mL, a decrease of 47.3%), and IL-10 (decreased from 295 ± 86 to 110 ± 31 pg/mL, a decrease of 62.7%) levels.
| Groups | Cytokine secretion levels | |||
|---|---|---|---|---|
| IL-1β (pg/mL) | TNF-α (pg/mL) | IL-6 (µg/mL) | IL-10 (pg/mL) | |
| OVA/COR (n = 7) | 35.6 ± 5.9B | 315 ± 57B | 4.13 ± 0.82 | 295 ±86B |
| OVA/COR-PER (n = 6) | 13.2 ± 2.3A | 166 ± 7A | 2.37 ± 0.37 | 110 ± 31A |
| OVA/PER (n = 7) | 20.0 ± 3.1A | 194 ± 17A | 3.33 ± 0.31 | 137 ± 15AB |
| PBS/COR (n = 7) | 39.5 ± 6.9 | 237 ± 25 | 1.56 ± 0.33* | 152 ± 17 |
- Values are means ± SEM. Values within the same column not sharing common capital letters are significantly different (p<0.05) from each other among ovalbumin (OVA)-challenged groups analyzed by ANOVA, followed by Duncan's multiple range test. The sensitivity of the ELISA kits used in this study was about <15.6 pg/mL. The lung cell density was 1 × 106 cells/mL medium; the lipopolysaccharide (LPS) concentration was 5 µg/mL. Asterisk (*) means significantly (p<0.05) different between OVA/COR and PBS/COR groups within same column analyzed by ANOVA, followed by unpaired Student's t-test.
Decreased IL-1β and TNF-α secretions in LPS-stimulated lung cells suggest that dietary PER may protect the lungs from inflammation during allergic asthma. However, the compound oil containing PER and COR seems to have a better anti-inflammatory ability through markedly decreasing IL-1β and TNF-α secretions as compared to those of dietary PER alone. Unfortunately, the dietary compound oil also significantly (p<0.05) decreased the secretion of an anti-inflammatory IL-10 cytokine. IL-10 can be expressed in different cells including monocytes, type 2 helper T cells (Th2 cells), mast cells, CD4+CD25+Foxp3+ regulatory T cells, and also in a certain subset of activated T cells and B cells. Cytotoxic T cells (CD8+ T cells) may produce IL-10 to suppress the actions of natural killer (NK) cells during viral infection. However, IL-10 is generally synthesized after TNF-α as well as when IL-1β increases. IL-10 administration inhibits TNF-α and IL-1β, but not IL-6 secretions by LPS-stimulated mouse peritoneal macrophages 22. Therefore, the LPS-stimulated lung cells need not produce extra IL-10 to inhibit extremely decreased TNF-α and IL-1β levels (Table 1). Due to extremely low TNF-α and IL-1β levels in the cultures, low IL-10 levels in the LPS-stimulated lung cell cultures were reasonable and suggested a lower inflammation status in the cell cultures. Our results suggest that dietary perilla compound oil may have strong protective effects on the lungs in chronic asthmatic subjects.
Undoubtedly, dietary oil patterns affected the cytokine secretions by primary lung cells from OVA-sensitized and -challenged mice through 5 wk feeding in the present study. Recently, supplementation with fish oil, which rich in n-3 PUFAs including ALNA (18:3n-3, 2.63%), eicosapentaenoic acid (EPA, 20:5n-3, 17.77%), and DHA (22:6n-3, 10.49%), inhibited the secretion of pro-inflammatory mediators, such as nitric oxide (NO), TNF-α, IL-1β, and IL-6, by LPS-stimulated murine peritoneal macrophages isolated from mice fed the fish oil-enriched diet for 8 wk 23. A recent study indicated that dietary n-3 fatty acids are incorporated into the phospholipid bilayer of cell membrane and modulate the intracellular signaling pathway of transcription factor and nuclear factor (NF)-κB 24. Toll like receptor (TLR)-4 is a pattern-recognition receptor for LPS on the cell surface to initiate intracellular inflammation signaling. TLR4-mediated downstream signaling pathway activates the transcription factor NF-κB to induce the expression of target genes, such as cyclooxygenase (COX)-2 and pro-inflammatory cytokines 25. Our results suggest that the dietary PER or its compound oil, which rich in n-3 PUFAs (ALNA-rich, but not EPA or DHA), may inhibit the production of pro-inflammatory cytokines by LPS-stimulated lung cells, possibly through affecting cell membrane composition and mediating a common signaling pathway from TLR4 (Table 1). However, the regulatory mechanism of dietary PER or its compound oil on the lung cells remains to be further studied.
Effects of dietary oils on the Th1/Th2, anti- and pro-inflammatory cytokine levels in the LPS-stimulated BALF cell cultures from OVA-challenged mice
To unravel protective effect of different dietary oils on the airways in asthmatic subjects, Th1/Th2, anti- and pro-inflammatory cytokine levels in the LPS-stimulated BALF cell cultures from OVA-challenged mice were determined. The levels of Th1 (IFN-γ), Th2 (IL-5), pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6), and an anti-inflammatory cytokine (IL-10) in the LPS-stimulated BALF cell cultures from OVA-challenged mice fed different experimental diets through 5 wk are shown in Table 2. The spontaneous (no LPS stimulation in vitro) cytokine levels secreted by the BALF cells were too low to be detectable (data not shown). The results showed that the IFN-γ (a Th1 cytokine) level secreted by BALF cells in the presence of LPS markedly increased from 320 ± 126 to 642 ± 138 pg/mL (an increase of 100.6%) in the OVA/COR-PER group, suggesting that the compound oil containing PER and COR may have potential in treating the Th2-skewed allergic asthma. However, there were no significant differences in IL-5, IL-1β, TNF-α, IL-6, and IL-10 among experimental groups (Table 2).
| Groups | Cytokine secretion levels | |||||
|---|---|---|---|---|---|---|
| IFN-γ (pg/mL) | IL-5 (pg/mL) | IL-1β (pg/mL) | TNF-α (pg/mL) | IL-6 (µg/mL) | IL-10 (pg/mL) | |
| OVA/COR (n = 4) | 320 ± 126 | 30.5 ± 2.5 | 23.2 ± 11.2 | 1125 ± 354 | 14.9 ± 3.3 | 146 ± 70 |
| OVA/COR-PER (n = 4) | 642 ± 138 | 28.8 ± 6.5 | 23.8 ± 7.1 | 1368 ± 402 | 14.9 ± 3.0 | 102 ± 16 |
| OVA/PER (n = 7) | 222 ± 63a) | 52.2 ± 12.3 | 17.5 ± 3.9 | 995 ± 148 | 16.1 ± 1.1 | 81 ± 16 |
| PBS/COR (n = 4) | 104 ± 67a) | 35.5 ± 15.4 | 30.8 ± 15.8 | 1767 ± 681 | 14.0 ± 5.3 | 214 ± 176 |
- Values are means ± SEM. The sensitivity of the ELISA kits used in this study was about <15.6 pg/mL. The BALF cell density was 5 × 105 cells/mL medium; the LPS concentration was 5 µg/mL. Data within the same column were first analyzed by Kruskal–Wallis (KW) test. If the statistical probability p<0.05 analyzed by the KW test, Mann–Whitney U-test was further used for comparisons between two random groups.
- a) p<0.05 versus OVA/COR-PER group.
Effects of dietary oils on the Th1/Th2, anti- and pro-inflammatory cytokine levels in the splenocyte cultures from OVA-challenged mice
To evaluate the effects of different dietary oils on systemic immune response in asthmatic subjects, Th1/Th2, anti- and pro-inflammatory cytokine levels in the splenocyte cultures from OVA-challenged mice were determined. The levels of Th1 (IFN-γ), Th2 (IL-4), a pro-inflammatory cytokine (IL-6) and an anti-inflammatory cytokine (IL-10) in the splenocyte cultures in the absence or presence of LPS from OVA-challenged mice fed different experimental diets through 5 wk are shown in Table 3. The results showed that the secretion levels of IL-6 and IL-10 by the splenocytes in the absence of LPS were markedly (p<0.05) decreased by dietary PER and its compound oil (OVA/PER and OVA/COR-PER groups). The IL-6 level decreased from 29.8 ± 8.0 to 3.3 ± 1.2 pg/mL; the IL-10 level decreased from 38.0 ± 8.1 to 5.6 ± 2.7pg/mL. In addition, the IFN-γ, IL-6, and IL-10 levels in the LPS-stimulated splenocyte cultures were also markedly (p<0.05) decreased by dietary PER and its compound oil (OVA/PER and OVA/COR-PER groups). The IFN-γ level decreased from 133 ± 39 to 7.2 ± 4.1 pg/mL (a decrease of 94.6%). The IL-6 level decreased from 1198 ± 105 to 425 ± 20 pg/mL (a decrease of 64.5%). The IL-10 level decreased from 1813 ± 178 to 1146 ± 103 pg/mL (a decrease of 36.8%).
| Groups | Cytokine secretion levels (pg/mL) | |||
|---|---|---|---|---|
| IFN-γ | IL-4 | IL-6 | IL-10 | |
| Spontaneous | ||||
| OVA/COR (n = 8) | – | 6.7 ± 2.2 | 29.8 ± 8.0B | 38.0 ± 8.1B |
| OVA/COR-PER (n = 8) | – | 4.8 ± 1.5 | 3.3 ± 1.2A | 5.6 ± 2.7A |
| OVA/PER (n = 9) | – | 2.8 ± 0.8 | 7.4 ± 2.9A | 11.1 ± 3.6A |
| PBS/COR (n = 11) | – | 6.4 ± 2.6 | 11.8 ± 1.5 | 24.0 ± 5.5 |
| LPS-stimulated | ||||
| OVA/COR (n = 8) | 133 ± 39B | 35.9 ± 2.9 | 1198 ± 105B | 1813 ± 178B |
| OVA/COR-PER (n = 8) | 7.2 ± 4.1A | 29.3 ± 2.1 | 547 ± 74A | 1295 ± 151A |
| OVA/PER (n = 9) | 10.2 ± 5.4A | 31.3 ± 1.2 | 425 ± 20A | 1146 ± 103A |
| PBS/COR (n = 11) | 71.4 ± 19.6 | 35.6 ± 1.3 | 1179 ± 55 | 1436 ± 152 |
- Values are means ± SEM. Values within the same column in the absence or presence of LPS not sharing common capital letters are significantly different (p<0.05) from each other among ovalbumin (OVA)-challenged groups analyzed by ANOVA, followed by Duncan's multiple range test. The marker (–) means the cytokine level was not detected. The sensitivity of the ELISA kits used in this study was about <15.6 pg/mL. The spleen cell density was 5 × 106 cells/mL medium; the LPS concentration was 5 µg/mL. There are no significant differences between OVA/COR and PBS/COR groups within same column in the absence or presence of LPS analyzed by ANOVA, followed by unpaired Student's t-test.
The spleen containing 41.54% of B lymphocytes and 47.11% of T lymphocytes in mice 21 collects antigens from the blood and may adequately, but not completely, reflect the systemic immune response. The present study indicated that dietary PER alone and its compound oil significantly decreased IL-6 and IL-10 cytokine secretions by the splenocytes in the absence of LPS, as well as markedly decreased IFN-γ, IL-6, and IL-10 secretions in the presence of LPS (Table 3), suggesting that dietary PER alone and its compound oil have an anti-inflammatory effect systemically during allergic asthma. Unfortunately, the spleen still could not completely reflect systemic immune responses. Lymphocytes from mesenteric lymph nodes may be more eloquent to examine the allergen and pro-inflammatory specific cytokines production. The mesenteric lymph node is more important than the spleen in regulating gut immunity. Lymphocytes of mesenteric lymph nodes from OVA-challenged asthmatic mice fed PER remain to be further studied.
Effects of dietary oils on cytokine secretion ratios by the LPS-stimulated BALF cells and splenocytes from ovalbumin-challenged mice
To further unravel the effects of dietary oils on the Th1/Th2 balance, the Th1/Th2 cytokine secretion ratios in the LPS-stimulated BALF cells were determined and shown in Fig. 1A. The results showed that the Th1/Th2 (IFN-γ/IL-5) cytokine secretion ratio significantly (p<0.05) increased in the LPS-stimulated BALF cell culture from the OVA/COR-PER group (increased from 10.0 ± 3.5 to 25.3 ± 7.9 pg/pg, an increase of 153%). It is recognized that major subphenotypes of asthma are accompanied with Th2-driven inflammation 7; dietary or drug therapies aiming at Th2 lymphocytes and maintaining the Th1/Th2 balance in vivo may be effective in the Th2-driven asthma patients 8. Our results provided the evidences that the compound oil consisting of PER and COR may exert an effect on shifting the Th1/Th2 balance to the Th1 inclination in the LPS-stimulated BALF cells during allergic asthma.
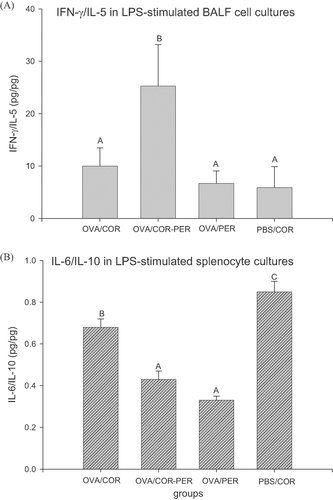
Effects of different dietary oils on secretion ratios of IFN-γ/IL-5 by (A) the LPS-stimulated BALF cells and (B) IL-6/IL-10 by the LPS-stimulated splenocytes from OVA-challenged mice through 5 wk feeding. Values are means ± SEM. Data of BALF cell cultures were first analyzed by KW test. If the statistical probability p<0.05 analyzed by the KW test, Mann–Whitney U-test was further used for comparisons between two random groups. Data of splenocyte cultures were analyzed by ANOVA, followed by Duncan's multiple range test. Bars within same plot not sharing common capital letters are significantly different (p<0.05).
Increased IFN-γ level (Table 2) and Th1/Th2 (IFN-γ/IL-5) cytokine secretion ratio (Fig. 1A) suggest that the compound oil (COR-PER) may modulate the Th2-skewed immune response in BALF cells. In our previous study, 5% dietary compound oil (COR-PER) administration significantly (p<0.05) decreased eosinophilic infiltration, but PER alone could not significantly (p>0.05) change the eosinophilic infiltration into the airways and the lungs 14. We supposed that the decreased eosinophilic infiltration might result from modulating the Th2-skewed immune balance toward the Th1 pole in the local tissues 14. This work and these findings were identical to the previous published report and further provided additional evidences that perilla compound oil modulated the Th2-skewed immune response in BALF cells. A high ratio of n-6/n-3 fatty acids in diet is a risk for allergic disease 26, however the optimal ratio of n-6/n-3 fatty acids is need for prevention and management of asthma 27. Based on the fatty acid composition profiles of PER and COR, the ratios of n-6/n-3 fatty acids in the experimental diets COR, PER, and COR-PER groups are 61.6, 0.27, and 1.02, respectively. A dietary intake of n-6 and n-3 PUFAs at a ratio of 4:1 or less has been recommended to avoid obesity and other chronic diseases 28, 29. This study further suggests that an appropriate n-6/n-3 fatty acid ratio at a ratio of 1:1 or less in dietary compound oil may be beneficial to mitigate the Th2-skewed allergic airway inflammation. A well-balanced n-6/n-3 fatty acid ratio from different vegetable materials may be developed for use by humans 30. Recent evidences indicated that lipid microdomains in the T-cell plasma membrane are remodeled by a dietary lipid consisting of n-3 PUFAs, EPA, and DHA 31. We supposed that the compound oil (COR-PER) might alter the lipid composition of BALF cell membrane. However, the cell membrane composition and cell signaling remain to be further studied.
To further unravel the effects of dietary oils on the pro-/anti-inflammatory cytokine secretion ratios, the ratios of pro-/anti-inflammatory cytokines (IL-6/IL-10) in the LPS-stimulated splenocyte cultures were determined (Fig. 1B). Figure 1B shows that dietary PER, whether PER alone (OVA/PER group) or its compound oil (OVA/COR-PER) markedly (p<0.05) decreased the IL-6/IL-10 secretion ratios in the LPS-stimulated splenocyte cultures (decreased from 0.68 ± 0.04 to 0.33 ± 0.02 pg/pg, a decrease of 51.5%). The results suggest that dietary PER alone or compound with COR, may exert an anti-inflammatory effect in the LPS-stimulated splenocytes via decreasing pro-inflammatory IL-6 levels, but comparatively increasing anti-inflammatory IL-10 levels. However, the IL-6/IL-10 secretion ratios in the LPS-stimulated lung and BALF cells did not significantly change (data not shown).
It is suggested that saturated fatty acids (SFAs) induce the expression of pro-inflammatory genes including COX-2, inducible nitric oxide synthase (iNOS), and IL-1α in macrophages (RAW264.7 cells), but unsaturated fatty acids (UFAs) including n-3 PUFAs (EPA and DHA) and n-6 PUFAs (linoleic acid) inhibit the expression of inflammatory genes 32. Lee et al. 32 suggested that both SFA-induced COX-2 expression and its inhibition by UFAs are mediated through a common signaling pathway derived from TLR-4 32. However, the possibility of TLR-4-mediated target gene expression and cellular responses are differently modulated by saturated (lauric acid) and unsaturated fatty acids (DHA) 33. DHA and EPA preferentially suppressed NF-κB activation that induced by LPS 34. The n-3 PUFAs (DHA and EPA) are better potent inhibitors of TLR-4 activation as compared to n-6 PUFAs (AA and linoleic acid) or n-9 UFA (oleic acid) 35. Recent studies demonstrated that n-3 PUFAs (DHA and EPA) were capable of inhibiting the LPS-stimulated inflammation in different cell types, including murine monocytic cell line (RAW 264.7) 34, murine bone marrow-derived dendritic cells 36, human blood monocytes 34, and human leukemia monocytic THP-1 cells 37. In the present study, our results further suggest that ALNA-rich PER may have similar effects to EPA and DHA on suppressing the inflammation induced by LPS in different organs and tissues of OVA-challenged mice. Dietary PER or its compound oil might modulate the splenocyte responses in the presence of LPS through remodeling the phospholipid composition of spleen cell membrane and/or acting as an antagonist against LPS action. This study further suggests that n-6/n-3 fatty acids at a ratio of 1:1 or less in dietary oil is beneficial to improve the Th2-skewed allergic asthmatic inflammation (Fig. 1A).
Conclusions
Overall, dietary PER diminished IL-1β and TNF-α secretions by the LPS-stimulated lung cells, as well as IFN-γ, IL-6, and IL-10 productions by the LPS-stimulated splenocytes from OVA-challenged mice. Dietary PER also increased Th1/Th2 (IFN-γ/IL-5) cytokine secretion ratio in the LPS-stimulated BALF cell cultures, but decreased IL-6/IL-10 (pro-/anti-inflammatory cytokines) secretion ratios in the LPS-stimulated splenocyte cultures. This study suggests that dietary PER may alleviate inflammation through inhibiting pro-inflammatory cytokine secretions in the airways, the lungs, and the spleen of OVA-challenged mice. This study further suggests that an appropriate n-6/n-3 fatty acid ratio of 1:1 or less in dietary oil may be beneficial to improve the Th2-skewed allergic asthmatic inflammation.
Acknowledgements
This study was kindly supported by a research grant NSC97-2321-B-005-004 from the National Science Council, Taipei, Taiwan, ROC.
The authors have declared no conflict of interest.




