Polyphenolic fractions improve the oxidative stability of microencapsulated linseed oil
Abstract
The main objective of this study was to evaluate the effect of incorporating polyphenolic-enriched fractions from murta leaves on the oxidative stability of linseed oil microencapsulated by spray drying. For this purpose, polyphenol-enriched fractions from murta leaves were separated by gel permeation chromatography and chemically characterized. The oxidative stability of microencapsulated linseed oil (MLO) with antioxidants was evaluated in storage conditions at 25°C for 40 days. The antioxidant effects of the polyphenolic fractions and commercial antioxidants (BHT and trolox) on microencapsulated oil were evaluated by the value of conjugated dienes, peroxide, and p-anisidine. In the initiation step of the oxidation, no significant oxidation delay (p>0.05) in MLO containing fractions F6, F8, or BHT and trolox was observed. However, in the termination step of the oxidation, the addition of fractions F6, F8, and BHT and trolox decreases significantly (p ≤ 0.05) the rancidity in MLO. Furthermore, the results of this study demonstrated the importance of the addition of natural antioxidants such as fractions of murta leaf extract in microencapsulated linseed oil to increase its resistance to oxidation.
Practical applications: For incorporating linseed oil, a source of omega-3 fatty acids, in the diet it is necessary to protect it against oxidative rancidity, the main cause of deterioration that affects food with a high unsaturated fat content. Microencapsulation is effective in retarding or suppressing the oxidation of unsaturated fatty acids and natural plants extracts are effective in inhibiting the lipid oxidation of microencapsulated oil. The use of process technology and a natural additive is expected to increase storage stability and enable its use in dry foods such as instant products. Linseed oil can be used in human nutrition as well as in animal feed as a replacement for fish oil.
Abbreviations:
CD, conjugated dienes; DPPH, 2,2-diphenyl-1-picrylhydrazyl; FC, Folin–Ciocalteu; MLO, microencapsulated linseed oil; p-AV, p-anisidine value; PV, peroxide value; TP, total polyphenol
Introduction
Lipids in seed oils are important functional components of foods and have a significant effect on their quality, although they constitute only a minor component. They not only contribute to flavor, odor, color, and texture, but also confer a feeling of satiety and palatability on foods. Linseed oil, also known as flaxseed oil, is a valuable source of n − 3 fatty acid. It is highly unsaturated (90% of total fatty acids), and particularly rich in ALA (C18:3n − 3), an important PUFA. It contains nearly 52–60% of ALA 1, 2, compared with 25% for plant oils generally. The ALA content is what makes it valuable as an essential component of healthy food. The scientific literature is full of evidence that suggests that regular consumption and/or dietary supplementation with long chain n − 3 PUFA gives several health benefits, including the prevention of cardiovascular diseases, inflammatory diseases, dyslexia, and depression 3. Potential mechanisms for the cardioprotective effect of n − 3 PUFAs include anti-arrhythmic, anti-inflammatory, hypotriglyceridemic effects, lowered blood pressure, and improved endothelial function 4. However, the main problem in the quality of oils lies in lipid oxidation during storage or food processing 5. Lipid peroxidation is one of the primary mechanisms of quality deterioration in foods. The changes in quality can be manifested by deterioration in flavor, color, texture, nutritive value, and the production of toxic compounds 5. Few studies address the effects of storage on linseed oil composition. In general, long-term storage and heat promote the auto-oxidation of fats, as well as decreases in the amounts of tocopherols and vitamin E in linseed oil 2. Many attempts have been made to prevent the oxidative deterioration of lipids by using natural antioxidants 6. Some components in natural products such as carotenoids, flavonoids, anthocyanins, and phenolic compounds are known to function as radical scavengers. In particular, the potential antioxidant activity of polyphenolic compounds has been reported for a number of natural plant extracts including grapes and their residues 7-9, almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster) 10, green teas 11, berries 12, and murta fruits and leaves 13, 14. Murta is a wild shrub growing in the Coast and pre-Andean mountains of southern Chile. The Mapuche, native inhabitants of the region, have passed down the health-beneficial properties of consuming its leaves for centuries. The infusions have been used traditionally to lessen urinary tract pain, and as astringents, stimulants, and phytoestrogenics 15. Murta extracts have shown high levels of flavonols like rutin and myricetin 13, substances with proven antioxidant activity, and to which the healthy properties of tea infusions are attributed. Although the antioxidant activity of different murta extracts has been extensively investigated 13, 14, 16, the separation of their antioxidant components in the extract by gel chromatography fractionation and the evaluation of the effect of polyphenol-enriched fractions in the stabilization of oils with high PUFA content have not yet been reported.
Another approach to protect lipid from oxidation is through microencapsulation, which has been widely used in manufacturing powder-type oil and fat products 17-19. Lipid microencapsulation offers several advantages: retarding auto-oxidation; enhancing stability; controlling lipid-soluble flavor release; masking the bitter taste of lipid soluble substances; and protecting dissolved substances against enzyme hydrolysis 20. Few studies have combined polyphenolic compounds and microcapsules for the prevention of lipid oxidation. It must be taken into account that microencapsulation of both linseed oil and polyphenolic antioxidants would make their consumption more efficient in terms of the availability of their beneficial properties.
The main objective of this study was to evaluate the effect of the incorporation of polyphenolic-enriched fractions from murta leaf on the oxidative stability of linseed oil microencapsulated by spray drying. As the first part of this study, a chemical characterization of fractions from murta leaf crude extract separated by gel permeation chromatography was performed. Then, the conditions for linseed oil microencapsulated by spray drying were defined, and the oxidative stability of linseed oil microencapsulated by spray drying in storage with and without antioxidant was subsequently evaluated.
Materials and methods
Raw material
Flaxseed was provided by Nutraseed Company. The oil was prepared from seeds (moisture content of 10%) by screw press. Then, the oil was centrifuged and stored in an amber glass bottle at 4°C until the preparation of emulsions.
Crude extract
Plant material (leaves) from murta was collected in January 2009 from the Andes mountains near the town of Villarrica in the Araucanía Region (Chile). The collected material was dried (at 35°C) to constant weight, ground in a coffee grinder, and sieved (Retsch, Germany). Samples (5 g) were macerated with ethanol (50% v/v in water, solvent-to-solid ratio of 5:1) using a mortar and pestle at RT and then filtered through Whatman No. 1 filter paper (Whatman International Ltd., Maidstone, UK). Filtrate was concentrated in a rotary evaporator at 35°C (Büchi R3000, Germany) and stored at −18°C until analysis. For the fractionation, the solid was dissolved in the same solvent that was previously used for extraction.
Fractionation of crude extracts in sephadex LH-20 column
Fractionation was performed using the method described by Jerez et al. 21. Extract of murta leaves (5 mL) was applied to a 50 cm × 2.5 cm id Sephadex LH-20 column. Sephadex LH-20 (50 g) was suspended in 50% methanol in water and allowed to swell for 24 h before the column was manually packed by elution with the same solvent. The column was eluted with 50% methanol in water at a flow rate of 4 mL/min. Samples were collected every 4 min using a fraction collector (Model 2110, Bio-Rad) and their absorbances were measured at 280 nm and plotted versus time. Fractions were collected and evaporated under vacuum to remove organic solvent, and dissolved in water (5 mL) to be stored at −18°C until analysis. The column was washed exhaustively with 70% acetone/water v/v to obtain the last fraction. All fractions were subsequently characterized according to its antioxidant activity and total polyphenol (TP) content.
Liquid chromatography-mass spectrometry (LC-MS-MS)
For the analyses of fractions, a Shimadzu Prominence HPLC system coupled to an Applied Biosystems 3200 QTRAP® LC/MS/MS system was used. An Inertsil ODS-3 RP-C18 column (2.1 mm × 150 mm × 4.6 mm) was used. Flow rate was set to 0.2 mL/min. The solvents used were 0.1% formic acid–water solution (A) and methanol (B). The elution gradient was as follows: 0–1 min, 95A/5B; 0.1–10 min, 95A/5B; 10–40 min, 5A/95B; 40–50 min, 5A/95B; 50–55 min, 95A/5B; 55–60 min, 95A/5B.
MS parameters were as follows: source temperature 380°C, voltage 4500 V, gas flow 20 L/min. The detection was carried out using multiple reaction monitoring (MRM) in negative ionization mode with analytes quantified from the summed total ion value of their most intense molecular ion transitions. The standard used were caffeic acid, gallocatechin gallate, epicatechin gallate, quercetin, kaempferol, luteolin, hesperetin, p-coumaric, (−)epicatechin, ferulic acid, vanillic acid, sinapic acid.
Partition coefficient of polyphenolic compounds
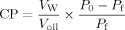
Evaluation of polyphenolic fractions from murta leaf on the oxidative stability of the emulsion
In order to select the more active polyphenolic fraction from murta leaf as an antioxidant in linseed oil, emulsions were prepared with each fraction as described above using as evaluation criteria the peroxide value (PV) and formation of conjugated dienes (CD) under accelerated storage at 50°C. Emulsions (60 g) were stored in open, amber glass beakers (200 mL). Samples were stored at 50°C in an oven without enforced circulation without light presence. Linseed oil without additive and crude extract from murta leaf was run in parallel.
Preliminary conditions for spray-drying microencapsulation
Stable emulsion of linseed oil/polysaccharide solution
Preliminary tests were conducted with different polysaccharides to obtain a stable emulsion of linseed oil/polysaccharide solution to be fed to the spray dryer. The polysaccharides tested as wall materials were potato starch, corn starch, maltodextrin, and arabic gum. These polysaccharides were selected according to results from previous reports. In fact, gum arabic, maltodextrin, and starch have been used as wall materials for food encapsulation as reported by Beristain et al. 17, Kenyon 23, Matsumara et al. 24, Fuchs et al. 25, among others. The assays were conducted using oil/polysaccharide proportions of, 1:1–1:4, 2:1–2:4, 3:1–3:4, in a homogenization range from 5000 to 45 000 × g, during 1–5 min of agitation. The stability of the resulting emulsions stored at 50°C for 16 h was evaluated visually examining the phase separation.
Conditions for the microencapsulation process by spray drying
The spray-drying process was carried out in a LabPlant SD-05 dryer (Huddersfield, England) on a laboratory scale, with a 1.5 mm diameter nozzle and a spray chamber of 500 mm × 215 mm.
To establish the conditions for the microencapsulation process by spray drying, preliminary tests were conducted using air input temperatures to the dryer (Ti) between 110 and 180°C, air outlet temperature from the dryer (Ts) from 75 to 125°C and feed temperatures (Tf) to the dryer from 20 to 40°C. Tests were conducted using a feed flow (Cf) to the dryer between 5.0 and 8.0 g/min.
Preparation of linseed oil emulsions for microencapsulation
Oil emulsions in water (oil/water) were prepared using gum arabic as wall material in a ratio of oil/gum arabic/water of 20/15/65 in 200 g of emulsion. The selected polyphenolic fractions with higher antioxidant activity were incorporated into linseed oil. Commercial antioxidants were added as a control of the evaluation of oxidation in the microencapsulation. BHT and trolox were dissolved in ethanol to be added to oil at a concentration of 2000 mg/L 26 and 613 mg/L, respectively 27. The concentration of fractions was 0.02 mg GAE/g linseed oil according to the ratio of antioxidant/linoleic acid of 1.0 × 10−4 to 1.0 × 10−2 w/w as reported by Fang et al. 28. The mixture was homogenized at 45 000 × g for 2 min.
Linseed oil was microencapsulated with and without antioxidants according to the conditions described previously. According to the methodology reported by Imagi et al. 29, samples as powder obtained after the spray-drying process were stored at −20°C in sealed plastic bags under vacuum for the oil stability study.
Determination of microencapsulation efficiency (ME)
For the determination of ME, it is necessary to quantify the total oil in the microcapsule and the free oil (known as the non-encapsulated oil fraction).
The determination of the total oil of microcapsules was performed according to the Association of Official Analytical Chemists 30. The Soxhlet extraction was accomplished in cellulose thimbles containing 2 g of dried samples. Extractions were carried out using 250 mL of petroleum ether for 5 h. The solvent was concentrated by vacuum rotary evaporation with a controlled temperature bath (40°C), then further dried in an oven at 105°C until constant weight.
According to Velasco et al. 31, the fraction of free oil was determined by adding 200 mL of light petroleum ether (60–80°C) to 4 g of microencapsulated powder, and stirring at RT for 15 min. After filtering and evaporating the solvent in a rotary evaporator, the extracted oil was dried until constant weight was attained.
The ME was calculated as reported by Velasco et al. 31 as follows: ME (%) = (microencapsulated oil (g)/total oil (g)) × 100
The microencapsulated oil amount was calculated from the difference between total oil and free oil amount.
Oxidative stability during the storage
The oxidative stability of linseed oil microcapsules (LOM) with antioxidants was evaluated in controlled storage conditions at 25°C for 40 days. Microcapsules (10 g) were stored in a open, amber glass beakers (25 mL) at 25°C in an oven without enforced circulation and without light presence. The antioxidant effects of the polyphenolic fractions and commercial antioxidants in microencapsulated oil were measured by the value of CD, PV, and p-anisidine value (p-AV). Microencapsulated oil without antioxidant was run as a control.
For these analyses, the extraction of oil from the microcapsules was carried out as described by Bligh and Dyer 32, with some modifications. Ten gram of homogeneous paste was extracted with 10 mL of chloroform and 20 mL of methanol stirring for 2 min and 30 s, respectively. A second extraction was performed where 20 mL of chloroform were added to the mixture and homogenized for 30 s. Then, the solution was diluted with water and homogenized during 30 s. The mixture was centrifuged at 2218 × g for 3 min at 4°C to separate phases. The chloroform was evaporated in a rotary evaporator at 40°C.
Analyses
Remaining moisture of murta leaves
The moisture was determined in murta leaves by the drying method to constant weight 30. The moisture content was calculated from the sample weight before and after drying at 105°C, in triplicate.
Determination of total polyphenols
Total phenolic content was determined in crude extract and fractions through reaction with FC reagent (Fluka, Japan), following the method reported by Velioglu et al. 33. Briefly, each solution (200 µL) was mixed with 1 mL of FC reagent (which had previously been diluted 10-fold with distilled water) and allowed to stand at 22°C for 5 min; 800 µL of Na2CO3 (60 g/L) (Sigma–Aldrich, Germany) solution were added to the mixture. After heating the tubes for 15 min at 45°C in a water bath, the tubes were allowed to cool in the dark for 30 min, after which the absorbance was measured at 765 nm (Genesys 6, Thermo Scientific, USA). The results were expressed as gallic acid equivalents per gram of dry mass (mg GAE/g dm).
2,2-Diphenyl-1-picrylhydrazyl (DPPH) method
The radical scavenging activity was determined, in crude extract and fractions, by using the stable, DPPH radical (Sigma–Aldrich, Germany) 34. An aliquot of each solution (3.2 mL) was mixed with 800 µL of 400 µM DPPH in ethanol. After 30 min of incubation in the dark, the absorbance was measured at 520 nm. The radical scavenging activity was obtained from interpolation into a calibration curve performed with Trolox (Calbiochem, Germany) solutions as standards (0–250 µg Trolox/mL). The antioxidant activity was expressed as IC50, which was defined as the final concentration of the tested sample required for the inhibition of the DPPH radical by 50%.
Oxidative stability of microencapsulated linseed oil
The oxidative stability was evaluated by means of CD, PV, and p-AV.
Conjugated dienes (CD)
The CD content in oil was determined by the method described by Cheung et al. 26. The sample (5 µL) was diluted in 5 mL of hexane. The absorbance of the solution was determined against a blank (hexane) at 234 nm. CD results were expressed in units of absorbance at 234 nm.
Peroxide value (PV)
The determination of the PV was performed according to AOAC methods 30 with some modification 35. Samples (2 g) were weighed into 250 mL Erlenmeyer flasks and 30 mL of 3:2 v/v acetic acid/chloroform mixture were added to the samples and the flasks were swirled to dissolve the samples. Aliquots of 0.5 mL of 2% KI solution were added to the samples and the reaction mixtures were left to stand for 1 min at RT with occasional shaking. Thirty (30) milliliters of distilled water were then added to the sample and the resulting reaction mixtures were each titrated with 0.1 N standardized sodium thiosulfate to a pale yellow color, then 2 mL of starch indicator were added and the titration was continued to a colorless end-point. A blank titration was carried out using a sample containing all the above reagents except oil sample.
p-Anisidine value
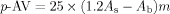
Statistical analysis
Experiments of microencapsulation were carried out at least in duplicate and results were expressed as mean values and their SDs. Assessments of oxidative stability of microencapsulated oil: DC, PV, and p-AV results were subjected an ANOVA of two factors (antioxidants and storage time). The difference between mean pairs was determined using Duncan's test with a probability of 5%.
Results and discussion
Total polyphenol content and antioxidant activity of fractions of murta leaf extract
The TP content and antioxidant activity of murta leaf extract were 50.54 ± 2.96 mg GAE/g dried leaf and 19.96 ± 0.370 TE/g dried leaf measured by DPPH method, respectively. Figure 1 shows the elution profiles which were obtained by measuring the absorbance of the fractions, obtained from the separation on Sephadex LH-20 column, measured at intervals of 4 min at 280 nm.
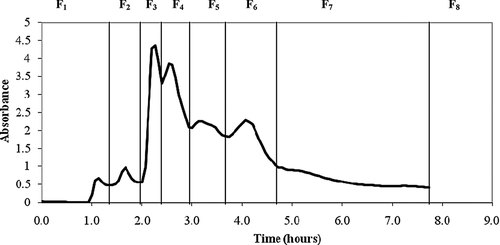
Chromatogram of crude extract of murta leaf. Flow: 1 mL/min.
Fractions under each peak were pooled in seven fractions, F1–F7. Washing with 70% acetone resulted in the recovery of fraction F8. Acetone was chosen because the carbonyl oxygen serves as a strong H-bond acceptor and allows displacement of bound polymeric phenols from Sephadex LH-20 21.
Sephadex LH-20 columns are useful to fractionate polyphenolic compounds based on molecular size and polarity; these are eluted with a mobile phase of alcohol–water or acetone–water 21.
In this study, the results showed that fraction F8 has the highest content of polyphenols and radical scavenging activity compared with other fractions, with a polyphenol recovery yield of 21.40% (Table 1). The lower TP content and radical scavenging activity were shown in fractions F1 and F2 with a polyphenol recovery yield of 0.04 and 0.16%, respectively (Table 1).
| Total polyphenol mg GAE/g dried leaf | Recovery yield (%) | Radical scavenging activity mg TE/g dried leaf | |
|---|---|---|---|
| Extract | 50.54 ± 2.96 | 100 | 19.96 ± 0.37 |
| F1 | 58.35 ± 2.98 | 0.04 | 4.63 ± 0.10 |
| F2 | 13.41 ± 0.04 | 0.16 | 4.65 ± 0.08 |
| F3 | 41.81 ± 0.30 | 0.81 | 20.28 ± 0.14 |
| F4 | 16.51 ± 3.64 | 0.26 | 45.77 ± 1.07 |
| F5 | 381.21 ± 1.04 | 1.30 | 188.09 ± 1.22 |
| F6 | 139.52 ± 1.01 | 0.65 | 66.09 ± 2.68 |
| F7 | 234.33 ± 1.14 | 0.49 | 63.55 ± 3.35 |
| F8 | 532.95 ± 1.91 | 21.15 | 306.00 ± 2.24 |
The first fractions of murta leaf extract reached the highest absorbance values at 280 nm compared with the last fractions, as was also shown for the separation of barley extract 37. Amarowicz et al. 37 obtained seven fractions from barley extract by Sephadex LH-20 column, with the last one presenting the highest TP content measured by the FC method as well as the highest radical scavenging activity measured by the method DPPH.
According to the results of polyphenol recovery yield regarding murta leaf extract, about 25% of polyphenols could be extracted by gel permeation chromatography. This recovery yield is lower compared with 50.1% of procyanidins obtained by Jerez et al. 21 in the fractionation of Pinus pinaster extract by Sephadex LH-20 column.
By comparing their mass spectral data and retention times with standards, phenolic compounds were identified in the fractions as shown in Table 2. The Extracted Ion Chromatogram (XIC) for each identified compounds in each fraction is shown in supplementary file.
| Fractions | m/z [M–H]− | m/z [M–H]− | Retention time (min) | Phenolic compound |
|---|---|---|---|---|
| Q1 | Q3 | |||
| 1 | 301 | 151–121 | 32.9–33.02 | Quercetin |
| 285 | 158–133 | 33.70 | Luteolin | |
| 301 | 164–151 | 33.8 | Hesperetin | |
| 2 | 179 | 135–107 | 24.14 | Caffeic acid |
| 457 | 169–125 | 24.80 | Gallocatechin gallate | |
| 441 | 289–169 | 25.85 | Epicatechin gallate | |
| 301 | 151–121 | 32.9–33.02 | Quercetin | |
| 285 | 143–117 | 34.90 | Kaempferol | |
| 285 | 158–133 | 33.70 | Luteolin | |
| 301 | 164–151 | 33.8 | Hesperetin | |
| 163 | 119–104 | 26.82 | p-Coumaric | |
| 3 | 179 | 135–107 | 24.14 | Caffeic acid |
| 457 | 169–125 | 24.80 | Gallocatechin gallate | |
| 301 | 151–121 | 32.9–33.02 | Quercetin | |
| 285 | 158–133 | 33.70 | Luteolin | |
| 163 | 119–104 | 26.82 | p-Coumaric | |
| 289 | 245–123 | 21.60 | (−)Epicatechin | |
| 4 | 179 | 135–107 | 24.14 | Caffeic acid |
| 193 | 134–178 | 27.56 | Ferulic acid | |
| 285 | 158–133 | 33.70 | Luteolin | |
| 163 | 119–104 | 26.82 | p-Coumaric | |
| 289 | 245–123 | 21.60 | (−)Epicatechin | |
| 5 | 265 | 152–108 | 23.85 | Vanillic acid |
| 179 | 135–107 | 24.14 | Caffeic acid | |
| 223 | 121–149 | 27.58 | Sinapic acid | |
| 193 | 134–178 | 27.56 | Ferulic acid | |
| 285 | 158–133 | 33.70 | Luteolin | |
| 163 | 119–104 | 26.82 | p-Coumaric | |
| 289 | 245–123 | 21.60 | (−)Epicatechin | |
| 6 | 265 | 152–108 | 23.85 | Vanillic acid |
| 179 | 135–107 | 24.14 | Caffeic acid | |
| 223 | 121–149 | 27.58 | Sinapic acid | |
| 193 | 134–178 | 27.56 | Ferulic acid | |
| 163 | 119–104 | 26.82 | p-Coumaric acid | |
| 289 | 245–123 | 21.60 | (−)Epicatechin | |
| 7 | 265 | 152–108 | 23.85 | Vanillic acid |
| 179 | 135–107 | 24.14 | Caffeic acid | |
| 223 | 121–149 | 27.58 | Sinapic acid | |
| 193 | 134–178 | 27.56 | Ferulic acid | |
| 285 | 158–133 | 33.70 | Luteolin | |
| 301 | 164–151 | 33.80 | Hesperetin | |
| 163 | 119–104 | 26.82 | p-Coumaric acid | |
| 289 | 245–123 | 21.60 | (−)Epicatechin | |
| 8 | 179 | 135–107 | 24.14 | Caffeic acid |
| 441 | 289–169 | 25.85 | Epicatechin gallate | |
| 301 | 151–121 | 32.90–33.02 | Quercetin | |
| 285 | 158–133 | 33.70 | Luteolin | |
| 163 | 119–104 | 26.82 | p-Coumaric acid | |
| 289 | 245–123 | 21.60 | (−)Epicatechin |
- m/z Q1: mass value unique on precursor (Q1). m/z Q3: mass value of fragment ion (Q3).
Evaluation of partition coefficient of fractions of murta leaf extract
The partition coefficient was evaluated for murta leaf fractions. It indicates the capacity of an antioxidant compound to remain in the aqueous or lipid phase of an emulsion which depends on its affinity with the phases or polarity 38.
Table 3 shows the percentage of TPs in the lipid phase after the partition and the partition coefficients for each fraction of murta leaf extract. The TP concentration in the lipid phase after the partition was 77.8 ± 0.18% in fraction F2 and 97.2 ± 0.05% in fraction F8. The partition coefficients were 3.5 ± 0.04 for fraction F2 and 34.2 ± 0.58 for fraction F8. As described by Huang et al. 22, these results show that fraction F2 is the most polar whereas fraction F8 is the most apolar. All factions presented a partition coefficient lower than the lipophilic fraction F8.
| Fractions | Total polyphenol (%) in lipid phase | Partition coefficient |
|---|---|---|
| F1 | 87.8 ± 0.29 | 7.2 ± 0.19 |
| F2 | 77.8 ± 0.18 | 3.5 ± 0.04 |
| F3 | 92.8 ± 0.12 | 12.8 ± 0.23 |
| F4 | 94.1 ± 0.43 | 16.1 ± 1.27 |
| F5 | 93.5 ± 0.05 | 14.4 ± 0.11 |
| F6 | 93.1 ± 0.07 | 13.4 ± 0.14 |
| F7 | 95.2 ± 0.00 | 19.8 ± 0.01 |
| F8 | 97.2 ± 0.05 | 34.2 ± 0.01 |
An antioxidant compound with lipophilic characteristics diffuses more freely to the lipid phase as the partition coefficient increases. This effect can be explained by the dissociation degree that depends on the antioxidant compound in water 38. Thus, a polar antioxidant compound diffuses more freely in the aqueous phase, so its partition coefficient is lower.
Evaluation of polyphenolic fractions from murta leaf on the oxidative stability of the emulsion
During the storage of emulsions containing polyphenolic fractions from murta leaf extract, lipid oxidation products such as CD and peroxide compounds were evaluated. Both compounds are known as the first stable products of oxidative damage and the source of unpleasant aromas and flavors 39.
Table 4(a) shows the formation of CD over 22 days. Regarding the emulsions containing fraction F1, F2, F5, or F8, the formation of these compounds increased rapidly along with the control sample. However, the emulsions containing fraction F3, F4, F6, or F7 were more stable during the storage time. Fraction F6 was the most active as an antioxidant in the emulsion showing a low CD value of 0.211 ± 0.02 at day 22. This value should indicate that the induction period of oil oxidation was slower with the addition of compounds present in this fraction.
| (a) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (days) | Conjugated dienes (absorbance) | |||||||||
| Crude extract | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | Linseed oil without additive | |
| 0 | 0.170 ± 0.007 | 0.205 ± 0.004 | 0.205 ± 0.004 | 0.205 ± 0.004 | 0.205 ± 0.004 | 0.205 ± 0.004 | 0.205 ± 0.004 | 0.205 ± 0.004 | 0.205 ± 0.004 | 0.205 ± 0.004 |
| 1 | 0.170 ± 0.004 | 0.215 ± 0.002 | 0.225 ± 0.017 | 0.222 ± 0.008 | 0.207 ± 0.012 | 0.182 ± 0.004 | 0.208 ± 0.005 | 0.106 ± 0.025 | 0.213 ± 0.001 | 0.231 ± 0.006 |
| 4 | 0.202 ± 0.004 | 0.299 ± 0.025 | 0.327 ± 0.022 | 0.281 ± 0.01 | 0.269 ± 0.015 | 0.27 ± 0.032 | 0.254 ± 0.01 | 0.273 ± 0.035 | 0.248 ± 0.021 | 0.273 ± 0.035 |
| 6 | 0.190 ± 0.008 | 0.232 ± 0.006 | 0.324 ± 0.015 | 0.19 5±0.009 | 0.191 ± 0.008 | 0.215 ± 0.001 | 0.195 ± 0.009 | 0.191 ± 0.007 | 0.225 ± 0.017 | 0.183 ± 0.016 |
| 9 | 0.169 ± 0.021 | 0.383 ± 0.033 | 0.439 ± 0.009 | 0.267 ± 0.052 | 0.214 ± 0.009 | 0.279 ± 0.033 | 0.211 ± 0.006 | 0.223 ± 0.023 | 0.246 ± 0.008 | 0.442 ± 0.027 |
| 15 | 0.244 ± 0.018 | 0.596 ± 0.044 | 0.640 ± 0.016 | 0.208 ± 0.009 | 0.189 ± 0.01 | 0.402 ± 0.016 | 0.191 ± 0.022 | 0.167 ± 0.007 | 0.398 ± 0.045 | 0.671 ± 0.025 |
| 18 | 0.255 ± 0.004 | 0.681 ± 0.108 | 0.761 ± 0.037 | 0.210 ± 0.017 | 0.171 ± 0.04 | 0.514 ± 0.013 | 0.199 ± 0.004 | 0.194 ± 0.004 | 0.596 ± 0.008 | 0.934 ± 0.002 |
| 22 | 0.437 ± 0.002 | 1.303 ± 0.122 | 1.057 ± 0.009 | 0.348 ± 0.013 | 0.227 ± 0.023 | 0.764 ± 0.006 | 0.211 ± 0.024 | 0.205 ± 0.001 | 1.181 ± 0.185 | 1.302 ± 0.015 |
| (b) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time (days) | Peroxide value (meq/kg) | |||||||||
| Crude extract | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | Linseed oil without additive | |
| 0 | 0.879 ± 0.014 | 0.879 ± 0.140 | 0.879 ± 0.140 | 0.879 ± 0.014 | 0.879 ± 0.140 | 0.879 ± 0.140 | 0.879 ± 0.140 | 0.879 ± 0.140 | 0.879 ± 0.14 | 0.879 ± 0.140 |
| 1 | 1.900 ± 0.294 | 1.963 ± 0.006 | 3.845 ± 0.106 | 1.762 ± 0.272 | 1.919 ± 0.054 | 1.92 ± 0.007 | 1.077 ± 0.134 | 0.877 ± 0.139 | 0.1960 ± 0.002 | 3.703 ± 0.294 |
| 4 | 2.345 ± 0.294 | 6.014 ± 0.244 | 9.646 ± 0.030 | 2.934 ± 0.004 | 3.913 ± 0.029 | 4.056 ± 0.284 | 1.895 ± 0.016 | 1.958 ± 0.005 | 3.867 ± 0.008 | 9.583 ± 0.219 |
| 6 | 3.627 ± 0.219 | 9.810 ± 0.004 | 22.56 ± 1.438 | 3.902 ± 0.021 | 4.362 ± 0.760 | 5.798 ± 0.101 | 2.644 ± 0.422 | 3.832 ± 0.117 | 4.872 ± 0.046 | 20.116 ± 2.058 |
| 9 | 3.660 ± 2.058 | 29.324 ± 0.067 | 42.108 ± 1.516 | 4.030 ± 0.411 | 5.659 ± 0.252 | 13.479 ± 0.373 | 3.241 ± 0.419 | 5.434 ± 0.571 | 7.378 ± 0.272 | 46.072 ± 1.417 |
| 15 | 6.309 ± 1.417 | 49.817 ± 1.228 | 62.795 ± 0.021 | 5.683 ± 0.302 | 6.022 ± 0.184 | 28.234 ± 1.627 | 4.107 ± 0.312 | 6.675 ± 0.286 | 31.542 ± 1.530 | 71.419 ± 1.115 |
| 18 | 16.155 ± 1.115 | 87.125 ± 1.080 | 83.173 ± 1.417 | 7.845 ± 0.018 | 6.339 ± 0.076 | 42.572 ± 0.627 | 5.888 ± 0.04 | 7.558 ± 0.010 | 62.983 ± 1.428 | 99.462 ± 1.072 |
| 22 | 32.551 ± 1.072 | 114.801 ± 1.245 | 126.086 ± 0.736 | 28.622 ± 1.403 | 11.234 ± 0.62 | 61.818 ± 1.258 | 10.732 ± 1.362 | 13.071 ± 2.002 | 98.764 ± 0.696 | 144.852 ± 1.88 |
Athukorala et al. 40 observed a higher inhibition of CD formation in fish oil with seaweed extract (Grateloupia filicina) used as the antioxidant, compared with synthetic antioxidants.
Table 4(b) shows the PV of linseed oil emulsion containing polyphenolic fractions of murta leaf extract, compared with the PV of linseed oil without additives (control) and with crude leaf extract. The emulsions containing F1, F2, F5, and F8 showed high values of PV compared with other fractions; however, the values were lower than the PV obtained for the oil without additive. However, the emulsions containing fraction F3, F4, F6, or F7 presented a low PV, and fraction F6 was the most active with a PV of 10.7 ± 1.36 meq/kg at day 22 of storage. Despite not having the highest polyphenol content, fraction F6 was the most active antioxidant in the emulsion, compared with the other fractions.
Polar paradox theory states that polar antioxidants are more effective in less polar media, such as bulk oils, whereas nonpolar antioxidants are more effective in relatively more polar media, such as oil-in-water o/w emulsions or liposomes 41. According to the polar paradox, oil-in-water emulsions, in contrast to bulk oils, are better protected from oxidation by nonpolar antioxidants than by polar ones. The higher effectiveness of nonpolar antioxidants in oil-in-water emulsions is primarily attributed to their greater affinity for the oil–water interface. Hydrophilic antioxidants are generally less effective in oil-in-water emulsions than lipophilic antioxidants because of their higher affinity toward the water phase. It should explain the high antioxidant activity of the apolar fraction F6 when it was incorporated into the emulsion. However, it would not explain the reduced activity of the apolar fraction F8. The action of antioxidants is possibly governed by more complex phenomena, and other factors such as molecular size, in addition to polarity, must be taken into account to explain efficiency.
Considering F6 and F8 are apolar nature with differences in antioxidant activity these fractions were selected for testing in the following experiments.
Conditions for the microencapsulation process by spray drying
To set the conditions for the microencapsulation process by spray drying, preliminary tests were conducted using air input temperatures to the dryer (Ti) between 110 and 180°C, air outlet temperature to the dryer (Ts) from 75 to 125°C and feed temperatures (Tf) to the dryer from 20 to 40°C.
The PV of microencapsulated linseed oil (MLO) was lower at input temperature of 140°C (1.9 meq/kg) than at 120 and 180°C (2.4 and 12.8 meq/kg, respectively). Furthermore, the selected input temperature for the dryer was 140°C, and the outlet temperature from the dryer was fixed at 95°C. The selected feed temperature was 20°C and the feed flow was fixed at 5.4 g/min. The emulsion was introduced into the main chamber through a peristaltic pump where the feed rate was controlled by the rotation of the speed pump. The drying air flow rate was 73 m3/h, and the compressor air pressure was 0.06 MPa. These conditions made it possible to obtain a microencapsulated dry product (not sticky) with a good performance of the spray dryer and were considered for the following steps.
The linseed oil emulsion in gum arabic aqueous solution selected as the most stable was atomized in a hot air stream supplying the drying chamber to produce the evaporation of water leading to the formation of microcapsules. Considering an average of total oil extracted from MLO of 1.1927 ± 0.0882 g (recovery yield of 60% using soxhlet extraction) and a free oil amount of 0.5403 ± 0.0032 g, the microencapsulated oil obtained from the difference of total and free oil was 0.6524 ± 0.0914. Finally, the ME obtained was 54.55 ± 3.61%.
Evaluation of polyphenolic fractions F6 and F8 in the oxidative stability of microencapsulated linseed oil
The antioxidant effect of fractions F6 and F8 in the MLO was compared with the antioxidant effect of commercial antioxidants such as BHT and trolox. Microencapsulated oil without antioxidant was run as a control. The formation of CD, PV, and p-AV under storage conditions at 25°C was assessed as an evaluation criterion.
Figure 2a shows the formation of CD over storage time in the MLO with antioxidants. The results show that fraction F8 accelerated the formation of CD from day 24 of storage, reaching 0.618 ± 0.030 at day 40. Fraction F6 and antioxidants BHT and trolox improve the stability to MLO showing a less formation of CD over time compared with the control. F6 was the most active as antioxidant compared with F8, presenting a CD value of 0.409 ± 0.052 after 40 days of storage, a result comparable with trolox and BHT, which reached CD values of 0.388 ± 0.057 and 0.338 ± 0.011 at day 40, respectively.
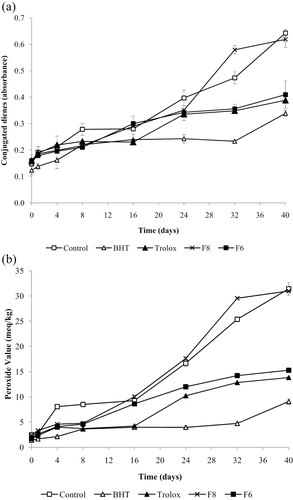
Antioxidant effect of fractions F6 and F8, BHT, and trolox on the formation of (a) CD and (b) peroxides in MLO.
Average values and SDs (±SD) were calculated. An ANOVA between antioxidants as well as between storage times was carried out, and Duncan's test was applied to determine the significance of variable association. The level of significance adopted in the statistical test was 95.0% (p ≤ 0.05). The oxidation of MLO with fraction F8 and the control was significantly higher (p ≤ 0.05) than with BHT. However, the control and MLO containing trolox, fractions F6 and F8 did not differ significantly (p ≤ 0.05) in the oxidation. Regarding the storage days, a lower oxidation of MLO was observed during the first 32 days compared with the MLO oxidation until 40 days of storage showing a significant difference in the oxidation (p ≤ 0.05).
The results show that MLO with fraction F8 accelerated the formation of peroxides (30.99 ± 0.343 meq/kg) during storage (Fig. 2b), a similar situation to the increase in the CD formation. The formation of peroxides in the control was similar (31.41 ± 1.27 meq/kg) at day 40. Fraction F6 was the most active as antioxidant between the tested fractions, with a PV of 15.26 ± 0.39 meq/kg at 40-day storage, comparable with the PV of trolox (13.82 ± 0.19 meq/kg) at day 40. MLO containing BHT presented the highest stability reaching 9.07 ± 0.53 meq/kg at day 40.
According to the ANOVA, significant differences (p ≤ 0.05) between antioxidants and between days of storage were observed. The results of Duncan's test determined the storage days and the type of antioxidant added to the MLO that showed significant differences in the oxidative stability of MLO. The oxidation of MLO containing fraction F8 and the control was significantly higher (p ≤ 0.05) than MLO containing antioxidants as BHT, trolox, and fraction F6. The MLO oxidation increased as the number of storage days increased, presenting a significant increase (p ≤ 0.05) at day 40 compared with the first 24 days of storage.
The p-AV is considered a robust indicator of the quality and degree of oxidation, detecting the end products of oxidation such as aldehydes and ketones, which are more stable than peroxides and CD produced at the initiation of the oxidation. The increase in p-AV implies an increased presence of secondary products of oxidation (rancidity). Figure 3 shows that the p-AV values increased during the storage.
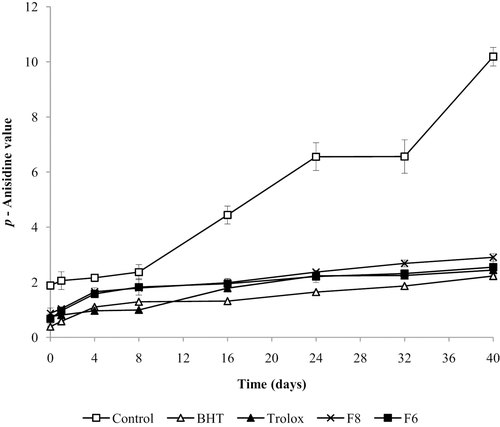
Antioxidant effect of fractions F6 and F8, BHT, and trolox on the formation of secondary products (p-AV) in MLO.
The results of p-AV indicated that the control showed an increase in the p-AV value from the first days of storage, reaching 10.19 ± 0.34 at day 40. Fractions F6, F8, and commercial antioxidants BHT and trolox delayed the rancidity in the MLO, obtaining p-AV values lower than the control. The incorporation of F6 in MLO delayed rancidity, obtaining a p-AV value of 2.55 ± 0.28 at day 40 of storage. Fraction F6 was more active than fraction F8, but fraction F6 was comparable with the values obtained for trolox (2.44 ± 0.17) and BHT (2.23 ± 0.13). These values indicate that the rancidity of microencapsulated oil with the addition of the commercial antioxidants and polyphenolic fractions was much slower in comparison with the control. Actually, at the final point of storage, the level of oxidation in microencapsulated oils did not allow a completely satisfactory comparisons between samples since values were low and they did not reach the end of the induction period.
However, the results of the ANOVA showed significant differences (p<0.05) between storage time and antioxidants on the p-AV value. Duncan's test determined the effect of storage time and each antioxidant on MLO. The results of Duncan's test indicated that the rancidity of the control was significantly higher (p ≤ 0.05) compared with MLO containing BHT, trolox and fractions F6 and F8. The rancidity of MLO containing antioxidants increased during storage. A significant increase of p-AV value (p ≤ 0.05) was observed at day 40 in comparison with the first 8 days of storage.
Furthermore, the results obtained at the initiation of lipid oxidation (assessed by CD and PV) showed that the commercial antioxidant BHT provided the highest antioxidant protection. Fraction F6 had a higher protective effect on MLO than fraction F8 during every day of storage and comparable with the antioxidant effect of the commercial antioxidant trolox in the MLO. In the termination step (as measured by p-AV), the oxidative rancidity in the MLO containing BHT, trolox, fractions F6 and F8 was significantly lower (p ≤ 0.05) compared with the control, and no significant differences (p>0.05) were observed between them. The MLO with BHT presented the lowest oxidative rancidity and fraction F6 was more active than fraction F8, with a stabilization of the rancidity of MLO comparable to trolox.
The polarity of phenolic antioxidants is an important parameter for determining their effectiveness in complex lipid systems such as microencapsulated oils.
In fact, the low polarity of an antioxidant and the size of its components seem to be important for an antioxidant to perform well in microencapsulated systems. Velasco et al. 27 assessed the effectiveness of phenolic antioxidants, structurally similar but different in polarity, in lyophilized pellets of sunflower oil previously stripped of their natural antioxidants under conditions of moderate temperature (40°C) and using copper (II) as an oxidation catalyst. Results showed that fewer polar antioxidants provided a higher protection. The polar antioxidants were probably located in the aqueous phase during preparation of the emulsion, not protecting the oil after drying. Recently, the efficacy of antioxidants with the same structure but different polarity has been studied in microencapsulated linoleic acid 28, 42 and microencapsulated fish oil 43. Results showed that lipophilic antioxidants studied were more effective than the hydrophilic antioxidants in total fat. In addition to the polarity, the structure of antioxidants (number of hydroxyl groups or chelating sites) also plays an important role in their action on lipid oxidation. Cuvelier et al. 44 observed differences in the inhibition of lipid oxidation because the antioxidants act according to their structure and their polarity.
In this study, the higher antioxidant activity of fraction F6 compared with fraction F8, both apolar fractions, could be attributed to the composition and molecular weight compounds contained in fraction F6. The molecular size of antioxidants may influence their effectiveness by decreasing their mobility in the multiphase system. Antioxidants with bulky structures, have lower mobility, because of steric hindrance, than those with a smaller size and, thus, decreased diffusibility toward the reactive centers, i.e., oxidizable substrates and free radicals 45.
The identification indicates that the composition of fractions F6 and F8 differs in the content of the flavonoids present in F8 and in the presence of greater number of cinnamic and benzoic acids detected in F6, which is basically composed of these compounds. Cinnamic and benzoic acids from F6 would have greater ease to reach the lipid–water interface where they must act, compared with the compounds from F8 where flavonoids have a higher contribution to the fraction (data not shown).
In conclusion, it must be pointed out that the results of antioxidant activity evaluated in the fractions using the DPPH method, were not consistent with the results of antioxidant activity that these fraction presented (formation of oxidation products such as DC, PV) when they were incorporated into the microencapsulated product. Therefore, it is important to note that assays on radical scavenging activity (in vitro analyses) were not representative of real oil oxidation.
It was found that fraction F6 of the murta leaf reached an increase in the oxidative stability of MLO with values comparable to those reached with trolox and BHT. In this fraction, vanillic, caffeic, sinapic, ferulic, p-coumaric acids, and (−)epicatechin were identified by LC-MS-MS. The high antioxidant activity of fractions and commercial fractions in microencapsulated systems was attributed mainly to their low polarity. According to the polar paradox, the use of lipophilic antioxidants is recommended for microencapsulation. The low polarity of an antioxidant seems to be crucial for a suitable antioxidant performance in microencapsulated systems. Furthermore, the results of this study demonstrated the importance of the addition of natural antioxidants in MLO and the possibility of using fractions of murta leaf extract to increase its resistance to oxidation.
On the other hand, the results presented for evaluation of lipid oxidation in microencapsulated oils correspond to overall oil. Although the data obtained are useful, further studies will be oriented toward differentiating between the oxidation of free and encapsulated oil for a better description of the oxidation process.
Acknowledgements
This research was supported by funding from Conicyt through Fondecyt project 1090516 and partially by GAP technical support provided by the Research Office at Universidad de La Frontera.
The authors have declared no conflict of interest.




