Utilisation of a national database to characterise renal function in patients with COVID19 infection
Abstract
Rationale
The incidence of renal function alterations among patients with COVID19 is unknown.
Objective
To determine the incidence of acute kidney injury (AKI) or augmented renal clearance (ARC) in patients hospitalised with COVID19 and identify risk factors for patients who may exhibit each renal alteration.
Methods
Retrospective, observational cohort analysis of hospitalise, adult patients within the National COVID Cohort Collaborative (N3C) database with laboratory confirmed COVID19 and available data to calculate creatinine clearance using the Cockcroft–Gault equation from 1 January 2020 through 9 April 2022.
Measurements
Incidence of AKI or ARC and patient demographics.
Main results
15 608 patients were included for renal function characterisation where 20.9% experienced AKI and 34.8% exhibited ARC. ARC lasted longer than AKI; however, AKI was associated with increased hospital length of stay and mortality. 11 274 patients were included in logistic regression analysis. Height and White race were the only variables associated with decreased risk of AKI while male sex and diabetes were associated with increased risk. Male sex, Black race and hypertension were associated with decreased risk of ARC. Age was associated with decreased risk of both AKI and ARC while weight and Hispanic ethnicity were associated with increased risk in both renal alterations.
Conclusions
A significant proportion of patients exhibit renal alterations during their hospitalisation for COVID19. These results provide initial evidence of identifying patients at risk of AKI or ARC, but more research is needed, especially with respect to use of biomarkers for renal alteration risk stratification.
1 INTRODUCTION
Since the first cases of COVID19, the virus has rapidly spread to become a pandemic infecting and hospitalising millions of patients with nearly one-third of patients admitted to the intensive care unit.1 While initially thought to affect mostly the lungs, it was quickly realised that COVID19 affected other organ systems as well including the brain, heart and kidneys.2 There have been multiple reports and reviews describing the development of acute kidney injury (AKI) and renal failure in patients infected with COVID19. It is estimated that 28% of all COVID19 hospitalised patients experience AKI, with higher prevalence in critically ill patients (46%).3 The development of a supraphysiologic degree of kidney function, known as augmented renal clearance (ARC), may also occur in patients with COVID19. A wide range, between 25 and 75%, of patients admitted for COVID19 have been reported to exhibit some degree of ARC.4-7 Both extremes in renal alteration could affect drug dosing and therapeutic effectiveness of renally eliminated medications. Therefore, it is important for clinicians to understand the existence and prevalence of both extremes in renal function to properly monitor drug therapy.
The pathophysiology of how COVID19 affects the kidney are not well defined. However, there are three proposed mechanisms by which the virus, specifically, is thought to interact with the kidneys including COVID19-induced coagulopathy, COVID19-induced inflammation and direct invasion of SARS-CoV-2 into the renal tissue.8 The results of COVID19-induced inflammation and direct viral invasion overlap with postulated mechanisms of ARC in patients without COVID19 including dysregulation of the renin–angiotensin–aldosterone system and increased production of inflammatory biomarkers, notably interleukins-6 and -8.8, 9 Given this crossover in hypothesised mechanisms, overlapping risk factors for patients at risk for developing AKI or ARC exist.
While there have been reports of isolated AKI or ARC, no single study to date has evaluated the full spectrum of renal function, nor have specific risk factors for AKI or ARC been fully elucidated in patients with COVID19. This study aimed to characterise renal function, including incidence of AKI, ARC and no alteration of renal function, in patients hospitalised with COVID19. Additionally, this study sought to determine specific risk factors for patients who may develop AKI or ARC.
2 METHODS
2.1 Data acquisition
This study utilised data from the National COVID Cohort Collaborative (N3C), an NIH National Center for Advancing Translational Sciences (NCATS)-sponsored data and analytic enclave which harmonises electronic health record data from over 70 sites in the United States.10 Harmonised, deidentified electronic health record data from the secured N3C data repository of patients with laboratory confirmed, possible or suspected COVID19 presenting after 1 January 2020 and uploaded to the Enclave before 9 April 2022 was used in this study. Data of interest were identified through Observational Medical Outcomes Partnership (OMOP) concept sets and concept IDs from ATLAS.11 The OMOP concept sets are lists of concepts standardised to describe a unique topic (e.g., hypertension) while ATLAS is a free, open-source software made available to the public by the Observational Health Data Science and Informatics community as a unified interface for patient level data and analytics compatible with N3C. The data used to generate and analyse this dataset in this current study are available in the NCATS N3C Data Enclave, https://covid.cd2h.org.
2.2 Patient identification
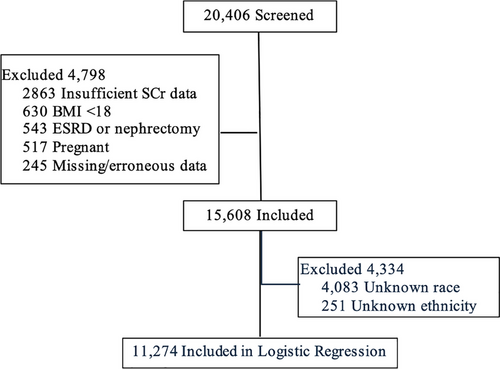
Patients were included if they were 18 years or older, admitted as an inpatient to a hospital and had laboratory confirmed (antigen or antibody positive) COVID19 and serum creatinine (SCr) data available. Patients were excluded if they were pregnant, had a history of nephrectomy or end-stage renal disease, body mass index less than 18 kg/m2, had fewer than 2 SCr values available to diagnose AKI, had missing data precluding creatinine clearance calculation (CrCl) or had significantly abnormal data thought to be at risk of skewing the data such as a patient height or weight resulting in a body mass index (BMI) of 4 or 400 kg/m2. This study was deemed exempt by the University of North Carolina at Chapel Hill institutional review board (IRB #21-0538:323480), accepted by N3C Data Access Committee (DUR-RP-B9DD40) and study results are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for cohort studies.12 In accordance with general best practices and to be as close to ‘real world’ practice, only patients with all relevant variables were included in the risk factor analysis and patients with ‘Unknown’ race or ethnicity were excluded.13
2.3 Definitions
AKI was defined using the KDIGO guideline criteria.14 Because of this definition, only SCr data from consecutive days from admission was used for analysis. Given the retrospective nature of this study, baseline SCr values for the majority of patients were not available. Therefore, the first SCr obtained within 24 h of a positive COVID19 laboratory test was considered ‘baseline’. ARC was defined as a CrCl ≥ 130 mL/min calculated via the Cockcroft–Gault equation.15 Finally, no alteration of renal function was defined as a patient not exhibiting AKI nor ARC during their hospitalisation. Notably, given the amount of missing discharge date data, length of stay (LOS) was calculated using the last SCr reported even if collected on a non-consecutive hospital day.
2.4 Data collection
Demographic data were collected including age, sex, height, weight, race, ethnicity and select comorbidities. Body mass index was calculated from height and weight data. With respect to renal function characteristics, average, minimum and maximum CrCl were determined for a patient's entire hospital stay and in addition to average, first, minimum and maximum CrCl for a patient's episode of altered renal function. Clinical outcomes including episode duration, hospital LOS and mortality were also collected. To assess possible risk factors for the development of either AKI or ARC, demographic variables in addition to laboratory values such as albumin, atrial natriuretic peptide, brain natriuretic peptide, C-reactive protein, cystatin c, d-dimer, ferritin, interleukins-6, -8, -18, neutrophil gelatinase-associated lipocalin and N-acetyl-glucosaminidase.
2.5 Statistical analysis
Descriptive statistics were used to examine demographic variables of all patients included in this study by renal characterisation assessment. Variable distributions of patients with either AKI or ARC were compared with those with no alteration of renal function using Wilcoxon Rank Sum test for continuous variables and chi-squared test for categorical variables. Clinical outcomes (LOS, mortality) were assessed similarly. To investigate potential risk factors for AKI or ARC, univariate simple logistic regression was conducted on all variables of interest. Multivariate logistic regression was then conducted including all variables found to be statistically significant (confidence interval not including one) in univariate logistic regression. A final multivariate model was determined using variables deemed statistically significant in the first multiple logistic regression. All analysis was conducted using R software within the Code Workbook of the N3C Enclave.
3 RESULTS
From 1 January 2020 until 9 April 2022, the N3C database had 4 942 399 COVID19 positive patients; 20 404 patients met screening criteria and 15 608 were included in the final analysis (Figure 1). Overall, 57.3% of patients were male of median age 62.7 [50.1–73.2] years (Table 1). Most patients were White (52.5%) and non-Hispanic (67%); however, 27.8 and 5.2% had unknown ethnicity and race, respectively. The majority of patients had hypertension (56.4%) while 35.7% had diabetes. The incidence of no alteration, AKI and ARC was 44.3, 20.9 and 34.8%, respectively. Patients who experienced AKI or ARC were younger than those with no alteration (65.6 [55.1–74.1] and 50.3 [38.7–60.2] vs. 70 [60.8–78.4], respectively; p < .001). While less White patients experienced AKI compared with no alteration (1535 (47.1%) vs. 3647 (52.8%); p < .001) patients of other and unknown race experienced AKI more frequently (115 (13.5%) vs. 163 (2.4%) and 988 (30.3%) vs. 1727 (25%); p < .001, respectively) (Table 1). Fewer Asian/Native Hawaiian/Pacific Islander and Black patients experienced ARC compared with no alteration (185 (3.4%) vs. 596 (8.6%) and 524 (9.6%) vs. 866 (12.5%); p < .001, respectively), whereas more White, other and unknown race experienced ARC (3017 (55.5%) vs. 3647 (52.8%), 187 (3.4%) vs. 163 (2.4%), and 1616 (27.1%) vs. 1727 (25%); p < .001, respectively) (Table 1). Patients with AKI had more comorbidities compared with no alteration while patients exhibiting ARC had less (Table 1). No patients included had documented chronic kidney disease within the database. With respect to severity of AKI, 77.1% had a maximum Stage 1 AKI followed by 12.8% with Stage 2 and 10.1% with Stage 3 (Table 1).
| Characteristic | Overall (n = 15 608) | No alteration (n = 6912) | AKI (n = 3260) | p Value* | ARC (n = 5436) | p Valuea |
|---|---|---|---|---|---|---|
| Age, median [IQR] | 62.7 [50.1–73.2] | 70 [60.8–78.4] | 65.6 [55.1–74.1] | <.001 | 50.3 [38.7–60.2] | <.001 |
| Male sex, No. (%) | 8937 (57.3) | 3594 (52) | 2101 (64.45) | <.001 | 3239 (59.58) | <.001 |
| Height (m), median [IQR] | 1.68 [1.6–1.78] | 1.68 [1.6–1.75] | 1.68 [1.63–1.78] | <.001 | 1.7 [1.63–1.78] | <.001 |
| Weight (kg), median [IQR] | 83.8 [70.6–100.2] | 76.1 [65.3–88.2] | 84.7 [71.5–102.8] | <.001 | 95.5 [81.6–113.4] | <.001 |
| BMI, median [IQR] | 29.3 [25.2–34.5] | 27 [23.8–30.9] | 29.6 [25.6–35.1] | <.001 | 32.7 [28.2–38.3] | <.001 |
| Race | ||||||
| Asian/Native Hawaiian/Pacific Islander, No. (%) | 1036 (6.6) | 596 (8.6) | 255 (7.8) | 0.11 | 185 (3.4) | <.001 |
| Black, No. (%) | 1825 (11.7) | 866 (12.5) | 435 (13.3) | 0.088 | 524 (9.6) | <.001 |
| White, No. (%) | 8199 (52.5) | 3647 (52.8) | 1535 (47.1) | <.001 | 3017 (55.5) | 0.002 |
| Other, No. (%) | 4653 | 163 (2.4) | 115 (3.5) | 0.008 | 187 (3.4) | 0.001 |
| Unknown, No. (%) | 4083 (26.2) | 1727 (25) | 988 (30.3) | <.001 | 1616 (29.7) | <.001 |
| Ethnicity | ||||||
| Hispanic, No. (%) | 4344 (27.8) | 1611 (23.3) | 972 (29.8) | <.001 | 1760 (32.4) | <.001 |
| Non-Hispanic, No. (%) | 10454 (67) | 4937 (71.4) | 2059 (63.2) | <.001 | 3459 (63.6) | <.001 |
| Unknown, No. (%) | 8105 | 364 (5.3) | 2297 | 0.002 | 2174 | 0.001 |
| Comorbidities | ||||||
| Hypertension, No. (%) | 8803 (56.4) | 4358 (63.1) | 2139 (65.6) | 0.012 | 2306 (42.4) | <.001 |
| Diabetes, No. (%) | 5575 (35.7) | 2461 (35.6) | 1497 (45.9) | <.001 | 1617 (29.8) | <.001 |
- Abbreviations: AKI, acute kidney injury; ARC, augmented renal clearance; BMI, body mass index; IQR, interquartile range.
- a Compared with ‘no alteration’.
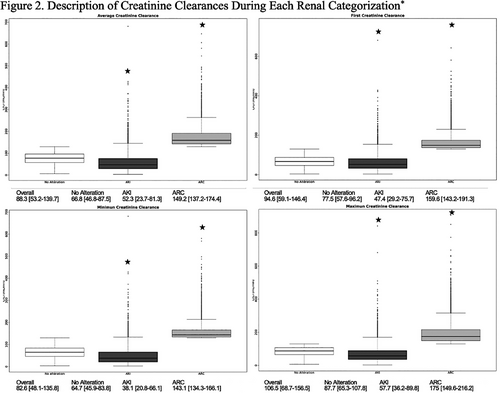
Table 2 displays average, minimum and maximum CrCl values for patients’ entire hospital stay as well as various clinical outcomes of interest. Of note, patients with AKI had higher maximum hospital stay CrCl compared with no alteration. As expected, average, first, minimum and maximum CrCls during an episode of AKI were all significantly lower compared with no alteration while patients with ARC had significantly higher CrCls (Figure 2).
| Outcome | Overall (n = 15 606) | No alteration (n = 6786) | AKI (n = 3579) | p Value | ARC (n = 5241) | p Value |
|---|---|---|---|---|---|---|
| Hospital Stay CrCl (mL/min), median [IQR]a | ||||||
| Average | 99.8 [65.7–142.1] | 77.5 [57.6–96.2] | 71.6 [41.9–112.6] | 0.011 | 154.5 [132.2–189.7] | <.001 |
| Minimum | 78.4 [46.8–116] | 64.7 [45.9–83.8] | 35.4 [19.9–61.9] | <.001 | 131 [108.5–162.6] | <.001 |
| Maximum | 117.9 [79–167.9] | 87.7 [65.3–107.8] | 105.7 [60.5–170.2] | <.001 | 175 [149.7–216.2] | <.001 |
| Episode duration (days), median [IQR]b | – | – | 3 [1–6] | – | 4 [2–7] | <.001 |
| Hospital LOS (days), median [IQR]a | 7 [4–14] | 6 [4–10] | 19 [10–34] | <.001 | 6 [4–11] | <.001 |
| Mortality, No. (%)a | 2354 (15.1) | 699 (10.1) | 1359 (41.7) | <.001 | 295 (5.43) | <.001 |
- AKI, acute kidney injury; ARC, augmented renal clearance; CrCl, creatinine clearance; IQR, interquartile range; LOS, length of stay.
- a Compared with ‘no alteration’.
- b AKI versus ARC.
From the 15 608 patients included in the renal characterisation analysis, 11 274 were included in the risk factor assessment analysis after excluding patients with ‘Unknown’ race or ethnicity (Table S1). Results of both univariate and multivariate logistic regression are shown in Table 3. Notably, no data were available for the selected laboratory risk factors from the N3C database. Compared with no alteration, patients who were male, Hispanic and had a history of diabetes were more likely to develop an AKI (1.1 (95% CI 1.07–1.13), 1.11 (95% CI 1.07–1.14), 1.06 (95% CI 1.04–1.08), respectively), while older age and White race were at decreased odds (0.996 (95% CI 0.995–0.997) and 0.969 (95% CI 0.948–0.991), respectively). Similarly, older age was associated with decreased odds of experiencing ARC (0.987 (95% CI 0.986–0.987) along with male gender (0.972 (95% CI 0.957–0.988), Black race (0.868 (95% CI 0.849–0.887) and a history of hypertension (0.954 (95% CI 0.939–0.97)). Hispanic patients were also more likely to develop ARC compared with no alteration (1.07 (95% CI 1.05–1.1)).
| AKIa | ARCa | |||||
|---|---|---|---|---|---|---|
| Univariate model | Multivariate model | Final multivariate model | Univariate model | Multivariate model | Final multivariate model | |
| Characteristic | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) | Odds ratio (95% CI) |
| Age, median [IQR] | 0.995 (0.994–0.996) | 0.996 (0.995–0.997) | 0.996 (0.995–0.997) | 0.984 (0.983–0.984) | 0.973 (0.972–0.974) | 0.973 (0.972–0.974) |
| Male sex, No. (%) | 1.12 (1.09–1.14) | 1.1 (1.07–1.13) | 1.1 (1.07–1.13) | 1.07 (1.05–1.09) | 0.947 (0.907–0.988) | 0.945 (0.916–0.976) |
| Height (m), median [IQR] | 1.48 (1.34–1.62) | 0.731 (0.641–0.833) | 0.729 (0.64–0.831) | 2.21 (2.02–2.41) | 0.995 (0.811–1.22) | – |
| Weight (kg), median [IQR] | 1.01 (1.01–1.01) | 1.01 (1–1.01) | 1.01 (1–1.01) | 1.01 (1.01–1.01) | 1.01 (1.01–1.02) | 1.01 (1.01–1.02) |
| Race | ||||||
| Asian/Native Hawaiian/Pacific Islander, No. (%) | 0.99 (0.958–1.02) | – | – | 0.811 (0.782–0.841) | 0.932 (0.841–1.22) | – |
| Black, No. (%) | 1.03 (0.997–1.06) | – | – | 0.943 (0.917–0.971) | 0.745 (0.677–0.819) | 0.753 (0.721–0.787) |
| White, No. (%) | 0.971 (0.949–0.993) | 0.969 (0.946–0.991) | 0.969 (0.948–0.991) | 1.11 (1.08–1.13) | 0.995 (0.913–1.09) | – |
| Other, No. (%) | 1.12 (1.06–1.18) | 0.99 (0.933–1.05) | – | 1.12 (1.06–1.18) | NA | – |
| Hispanic, No. (%) | 1.13 (1.09–1.16) | 1.11 (1.07–1.14) | 1.11 (1.07–1.14) | 1.13 (1.1–1.17) | 1.07 (1.04–1.1) | 1.15 (1.1–1.2) |
| Comorbidities | ||||||
| Hypertension, No. (%) | 1.03 (1.01–1.06) | 1.01 (0.99–1.03) | – | 0.836 (0.82–0.853) | 0.913 (0.882–0.945) | 0.91 (0.881–0.942) |
| Diabetes, No. (%) | 1.1 (1.08–1.13) | 1.06 (1.03–1.08) | 1.06 (1.04–1.08) | 0.953 (0.933–0.975) | 0.997 (0.963–1.03) | – |
- AKI, acute kidney injury; ARC, augmented renal clearance; CI, confidence interval; IQR, interquartile range; m, meter; kg, kilogram.
- a Compared with ‘no alteration’.
4 DISCUSSION
This population level assessment of renal function in patients hospitalised with COVID19 from the N3C database indicate that a significant number of patients experience alterations in renal function, either dysfunction (AKI) or enhancement (ARC) during their hospitalisation. Both types of alteration are associated with longer hospital LOS while patients who develop AKI are more likely to die during their hospitalisation. Described risk factors for both alterations are similar. Specifically, this study found that younger age, increasing weight and Hispanic ethnicity were associated with both AKI and ARC. Male sex was the only variable in the multivariate logistic regression to have increased risk of AKI but decreased risk of ARC.
This is the largest population level analysis investigating differences in renal function in COVID19. This is also the first study to assess the spectrum of renal function including impaired to augmented CrCl. The results indicate the incidence of AKI is similar to, but slightly less than, that reported in previous literature (22.9 vs. 28–36.4%).8, 16 The incidence of ARC is within the range of previously published literature with nearly one-third of patients hospitalised with COVID19 experiencing supraphysiologic renal function. In fact, these results indicate that patients are more likely to exhibit augmentation in their renal function than impairment. This finding could have implications for dosing renally eliminated medications. Importantly, ARC in patients with COVID19 has been associated with difficulty achieving therapeutic vancomycin concentrations and increased incidence of deep vein thrombosis or pulmonary embolism with subtherapeutic anti-Xa level despite therapeutic heparin or low-molecular-weight heparin dosing.6, 7
Much research has been devoted to studying AKI in hospitalised patients, including those with COVID19. Identified risk factors for the development of AKI include male sex, increasing age, presence of chronic comorbidities including chronic kidney disease, diabetes and hypertension.17 Specifically in patients with COVID19, Black race has also been noted to be a risk factor for AKI.18 While less research has been conducted on ARC, notable risk factors have included male sex, younger age, trauma admission, fewer comorbid conditions and lower severity of illness.8 Results from this study, however, differ from those reported in previous literature. Male sex was still determined to be a risk of AKI; however, female sex had higher risk of exhibiting ARC, contrary to previous literature. The most notable deviation from previously published literature was the overlapping association with younger age and both AKI and ARC, albeit a higher association with ARC than AKI. A possible explanation for this conflicting result is the fact that in the present study the risk of developing AKI was compared only with patients who had no alteration in their renal function and patients with ARC were not included. Other studies have compared AKI with all others, including those with ARC who are known to be younger in general because of their postulated increased renal reserve compared with older patients which would skew the risk assessment.3, 19 It is possible that, if the 5436 patients with ARC were added to the 6912 patients with no alteration, the average age of the group would decrease significantly, thus resulting in older age as a risk factor for AKI. This finding warrants further exploration to assess if the presence of ARC is influencing the association of age and AKI in patients with COVID19. Other overlapping risk factors included increased weight and Hispanic ethnicity. Morbid obesity has been shown to be a risk factor for AKI in morbidly obese patients in Mexico with COVID19, possibly due to increased oxidative stress and inflammation, which supports weight and Hispanic ethnicity risk factors identified in this study.20 Additionally, since weight is directly proportional to CrCl when estimated via the Cockcroft–Gault equation, it is not surprising that this risk factor is also associated with ARC. Moreover, pathophysiological increases in cardiac output and renal blood flow support increased weight as a risk factor for ARC as well.21 This effect of weight on ARC is mitigated; however, given patients who met AKI criteria regardless of estimated CrCl were classified as having AKI and not ARC. Notable risk factors for AKI included non-white race, although no specific race (Asian/Native Hawaiian/Pacific Islander, Black or Other) were individually associated with AKI, and patients with a history of diabetes, similar to previous literature.17 Demographics associated with ARC included absence of hypertension and non-Black race, again with an absence of other specific races as positive indicators. While these identified demographic risk factors provide some context for clinicians, none have strong associations and many overlap with either end of the spectrum of renal function. Clinical data including laboratory variables could provide more evidence to help clinicians predict which patient may shunt towards decreased versus increased renal function. These data variables, however, were not available in the present study yet still warrants consideration in future studies.
This study has notable limitations. First, although a national database consisting of over four million patients, only 15 608 patients from 11 of 72 sites met predetermined inclusion/exclusion criteria; thus, these results may not be generalisable to the entirety of patients hospitalised with COVID19. This study utilised strict inclusion/exclusion criteria, however, to understand the degree of renal alterations more fully, including severity of AKI which has not been regularly reported. Second, several key laboratory and measurement values were not uniformly available or reported. While efforts have been made to harmonise and unite the data by N3C and the investigators, no desired laboratory biomarker value was available for any patient among the 11 included sites, severely limiting the ability to determine patients who may be at risk for experiencing an alteration in renal function. Second, no patients were identified to have had CKD, a well-described risk factor for AKI, possibly in discordance in concept IDs/concept sets utilised. There were likely patients included in this cohort that did have CKD and future research should include this variable to better describe the risk of AKI development in patients with CKD that are infected with COVID-19. Third, a limit on CrCl was not placed on patients who were determined to have experienced AKI. Therefore, patients could have been eligible for inclusion in either cohort, AKI or ARC. Because of the more severe consequences of experiencing AKI patients who were eligible for either cohort were assigned to the AKI cohort. This could confound the results of the AKI cohort relative to the ARC cohort as these patients may behave, clinically, more similar to ARC. However, this accounts for a small number of patients compared with the overall included population and likely does not contribute significantly to the overall results of this study. Similarly, discharge dates were not available for the vast majority of patients in the present study resulting in last SCr measurement to be used as a surrogate for hospital LOS. The use of this surrogate may falsely lessen the LOS for patients with ARC or no alteration if they may be less likely to receive blood draws and a SCr checked. Finally, the use of SCr as the marker of renal function is not optimal; however, it more reflects real world practice in both intensive care units and acute care units in the hospital setting. While some institutions will obtain measured urine creatinine clearance, which is needed for definitive definition of ARC, it is not standard of care. The use of SCr is therefore more applicable to the majority of clinicians for most patients. It should be noted, however, that confirmation using either measured urine creatinine clearance or correlating with urine output is recommended.
Renal function derangements are common in patients with COVID19, which may impact hospital LOSs and mortality. It remains unclear, however, which patients may exhibit either AKI or ARC from baseline demographic variables alone. It is critical that clinicians are mindful of the possibility of both AKI and ARC. Additionally, future research should investigate the impact of both AKI and ARC on drug efficacy in patients hospitalised with COVID19.
AUTHOR CONTRIBUTIONS
N. N. and D. R. contributed to study conception. N. N., N. F. and D. R. contributed to study design and analysis. N. N. drafted the manuscript. N. N., N. F. and D. R. critically revised the manuscript, gave final approval and agree to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave https://covid.cd2h.org and N3C Attribution & Publication Policy v 1.2-2020-08-25b supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data and the organisations (https://ncats.nih.gov/n3c/resources/data-contribution/data-transfer-agreement-signatories) and scientists who have contributed to the on-going development of this community resource [https://doi.org/10.1093/jamia/ocaa196]. This research was also funded in part by the American College of Clinical Pharmacy—Critical Care Practice and Research Network. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH. The authors would like to thank Reema Thakkar for her assistance completing the statistical analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was deemed exempt by the University of North Carolina at Chapel Hill institutional review board (IRB #21-0538:323480), accepted by N3C Data Access Committee (DUR-RP-B9DD40).
Open Research
DATA AVAILABILITY STATEMENT
The data used to generate and analyse this dataset in this current study are available in the NCATS N3C Data Enclave, https://covid.cd2h.org.




