Molecular mechanisms underlying oesophageal cancer development triggered by chronic alcohol consumption
Abstract
This review explores the mechanisms underlying alcohol-induced oesophageal carcinogenesis, including DNA damage, oxidative stress, and nutritional deficiencies. Alcohol metabolism primarily involves alcohol dehydrogenase (ADH) converting ethanol to acetaldehyde, which can cause DNA damage, inhibit repair mechanisms, and form DNA adducts thus inhibiting DNA replication. Plus, it delves into the epidemiological evidence, genetic susceptibility, epigenetic modifications, biomarkers, and preventive strategies associated with alcohol-related oesophageal cancers. Consumption of alcohol increases the risk of gastroesophageal reflux disease thus compromising mucosal integrity of the oesophagus as dysregulation of cytokines such as IL-18, TNFA, GATA3, TLR4, and CD68 expands the intercellular spaces of epithelial cells. Genetic variants, such as ADH1B rs1229984 and ALDH2 rs671, significantly influence susceptibility to alcohol-related oesophageal cancers, with these variations affecting acetaldehyde metabolism and cancer risk. Understanding these factors is crucial for early detection, effective treatment, and the development of targeted prevention strategies. Biomarkers, such as miRNA and metabolite markers, offer non-invasive methods for early detection, while advanced endoscopic techniques provide better diagnostic accuracy. Pharmacological interventions, such as statins and proton pump inhibitors, also show potential for reducing cancer progression in high-risk individuals. Despite advances, late-stage oesophageal cancer diagnoses are still common, highlighting the need for better screening and prevention. Further research, including this study, should aim to improve early detection, personalise prevention, and explore new treatments to reduce cases and enhance outcomes in alcohol-related oesophageal cancers.
1 INTRODUCTION
1.1 Overview of oesophageal cancers
Around 600 000 global cases of cancer per year are attributed to oesophageal cancer, the seventh most common and sixth leading cause of cancer-related deaths worldwide. Oesophageal cancer arises when cancerous cells start to develop in the oesophagus lining. It can be classified historically into two main types: oesophageal squamous cell carcinoma (ESCC), which originates from stratified squamous epithelium, and oesophageal adenocarcinoma (EAC), from columnar glandular epithelium. ESCC impacts the majority of the human population, accounting for 90% of the cases worldwide, particularly in developing countries, while EAC is most common in Europe and North America.1-4
Oesophageal squamous cell carcinoma, normally detected at the upper two-thirds of the oesophagus, is commonly linked to repeated exposure to alcohol and other risk factors, damaging the epithelium lining the oesophagus over time. Meanwhile, oesophageal adenocarcinoma typically occurs at the lower one-third of the oesophagus, near the gastroesophageal junction. EAC is often associated with obesity or the presence of chronic, severe gastro-oesophageal reflux disease and Barrett's oesophagus (BE), a condition where the squamous epithelium of the oesophagus undergoes intestinal metaplasia to form columnar epithelium, which is found in the intestines.3
The most common presenting symptom of oesophageal cancers is progressive dysphagia, which progresses from difficulty in swallowing solids to liquids. Diagnosis often includes gastroscopy, a form of endoscopy, with a biopsy of the upper gastrointestinal tract (oesophagus, larynx, trachea, and bronchi), and imaging scans. It generally has a poor prognosis as patients are generally diagnosed at late, advanced stages. This is because most patients experience no symptoms initially. Treatment options comprise surgery, chemotherapy, radiotherapy, immunotherapy, or a combination of these, depending on the stage and grade of the cancer. In this review, we will delve into oesophageal cancers induced by chronic alcohol consumption, which is mainly ESCC, outlining its prevalence, epidemiology, mechanisms of carcinogenesis, pathogenesis, genetic factors, epigenetic modifications of chronic alcohol consumption, diagnosis, treatment, and prognosis. We also suggest some prevention strategies in terms of lifestyle modifications and pharmacological interventions in high-risk populations, along with recommendations for public health interventions and policies.
1.2 Prevalence of oesophageal cancers in the context of alcohol consumption
In 2020, the age-standardised rate of oesophageal cancer was 6.3 per 100,000 population.5 The US National Cancer Institute proposed that approximately 0.5 percent of the US population will be diagnosed with oesophageal cancer throughout their lifetime, according to data from 2018 to 2021, excluding 2020 due to COVID-19. Whereas American Cancer Institute calculated the lifetime risk of getting oesophageal cancer in the US is around 1 in 127 for males and 1 in 434 for females. According to global aggregate data on worldwide cancer incidence, an estimated 604 100 cases of oesophageal cancer were diagnosed in 2020, amounting to roughly 3% of all cancer cases. A total of 512 500 cases (85%) were ESCCs and 85 700 cases (14%) were EACs.6 Among all new cancer cases reported in 2020 worldwide, 741 300 cases were estimated to be caused by alcohol consumption. ESCC contributed the most cases (n = 189,700).
According to the US National Cancer Institute, as of 1 January 2021, 51 185 people in the United States were estimated to be living with oesophageal cancer. Meanwhile, the rate of incident cases of oesophageal cancer was estimated at 4.2 per 100 000 men and women per year. The death rate in 2021 was 3.7 per 100 000 men and women per year. These age-adjusted rates are determined based on cases between 2017 and 2021 and deaths between 2018 and 2022. A total of 22 370 new cases of oesophageal cancer are expected in 2024. Gender-based differences in the incidence of ESCC are found in low-incidence countries but not significant in high-incidence countries, likely attributed to the differences in underlying risk factors.7 Males account for the majority, or 69%, of new EC cases (n = 418 350), compared with females (n = 185 750).6, 8 Prevalence of EC is also found to be higher in patients with lower socioeconomic status.9
2 MECHANISMS OF CARCINOGENESIS
2.1 Role of ethanol in DNA damage and repair
The most common pathway for alcohol metabolism involves alcohol dehydrogenase (ADH) which converts ethanol to acetaldehyde. Acetaldehyde can directly attack DNA and cause damage in the form of DNA strand breaks, point mutations and micronuclei.10 Acetaldehyde can also bind to proteins and inhibit DNA repair mechanism by altering enzymes such as O6-methylguanine-DNA methyltransferase (MGMT) and 8-oxoguanine DNA glycosylase.11, 12 In addition, acetaldehyde can also react with DNA bases to form adducts. Unrepaired DNA adducts can inhibit DNA replication, promote sister chromatid exchange, and increase the chance of carcinogenesis.10 The most abundant DNA base adduct is N2-ethyl-2′-deoxyguanosine (N2-EtdG), which is derived from the reduction of N2-ethylidene-2′-deoxyguanosine (N2-EtidG) produced through the interaction between a single acetaldehyde molecule and deoxyguanosine (dG).10, 13 N2-EtdG can block replicative DNA polymerases α.13 To bypass the lesion, error-prone translesion synthesis (TLS) is involved, resulting mainly in frameshift mutations.13 The repair mechanism specific for N2-Et-dG is still uncertain.10 Another adduct that is less common but more mutagenic is crotonaldehyde-derived N2-propanodeoxyguanosine (Cr-PdG).13 Cr-PdG is formed from the interaction between dG and crotonaldehyde derived from two acetaldehyde molecules.10, 13 In a ring-closed state, DNA replication may be obstructed due to inhibition of base pairing with cytosine.10, 13 In a ring-open state, DNA-DNA interstrand and intrastrand crosslinks may be formed due to the active aldehyde group being exposed.10, 13 Nucleotide excision repair (NER) and translesion polymerase may be involved in Cr-PdG repair, whereas interstrand crosslinks are believed to be repaired via Fanconi anaemia (FA) pathway.10, 13
2.2 Oxidative stress and alcohol-induced oesophageal cancer
When a large amount of alcohol has been consumed, ethanol can also be metabolized through a different pathway involving the enzyme cytochrome P450 2E1 (CYP2E1). High CYP2E1 concentration generate high levels of reactive oxygen species (ROS) such as hydroxyl radical and superoxide anions that can react with various types of molecules.14 Hydroxyl radical can cause breakage of DNA strands and oxidise DNA bases to form mutagenic products such as 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG).13, 14 Interaction of ROS and lipid molecules causes lipid peroxidation.12, 14 Lipid peroxidation produces malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE).12, 14 They damage DNA bases by forming etheno adducts such as 1,N6-etheno-2′-deoxyadenine (NϵdA) and 3,N4-etheno-2′-deoxycytidine (NϵdC), which are primarily repaired by base excision repair (BER).13 Another etheno adduct, 1,N2-etheno-2′-deoxyguanosine (NϵdG), can block replicative DNA polymerase δ and the lesion is bypassed via TLS, which leads to possible mutagenic results.10, 13 Moreover, chronic alcohol consumption also stimulates the generation of nitric oxide, which induces the formation of peroxynitrite (ONOO−)15. Oxidative stress contributes to oesophageal cancer through oxidative injury, inflammation and other mechanisms that promote mutations.
2.3 Interplay between alcohol and nutritional deficiencies
Inflammation due to chronic alcohol consumption causes physical discomfort and increased cytokines production that may lead to poor appetite.16 Furthermore, chronic alcohol consumption can damage epithelial tissues in the stomach and small intestines and change the gut microbiome.16 As a result, normal absorption of nutrients in the gastrointestinal tract is disrupted. In addition, chronic alcohol consumption interferes with the body's metabolism and affects the body's ability to absorb, break down and utilise the nutrients even if the drinker follows a balanced diet.
Alcohol provides “empty” calories because it contains little vitamins, minerals and proteins, affecting the nutritional quality of diets.17 Excessive intake of alcohol has a significant effect on macronutrients and micronutrients because the percentage of energy derived from protein, fat and carbohydrate declines.17 In terms of carbohydrates, binge drinking leads to hypoglycaemia due to gluconeogenesis and glycogenolysis being inhibited.16 Prolonged alcohol consumption is linked to protein deficiency because it may affect the uptake of essential amino acids and disrupt protein synthesis from the liver.18 Furthermore, ethanol may increase protein requirement because it can promote urinary loss of nitrogen as shown in rats.17 Lipid metabolism is also affected as alcohol inhibits beta-oxidation of fatty acids and promotes esterification, resulting in triglyceride accumulation in the liver.16 For micronutrients, vitamin deficiencies are particularly common among alcoholics due to oxidative injury.17, 18 The increased concentration of microsomal enzymes due to the activated microsomal ethanol oxidising system pathway can promote the breakdown of vitamin A.17 Moreover, as the acetaldehyde level increases, the degradation of vitamin B6 is also enhanced.17 Chronic alcohol consumption is also related to deficiencies in zinc, magnesium, calcium, and fibre16, 17 (Figure 1).
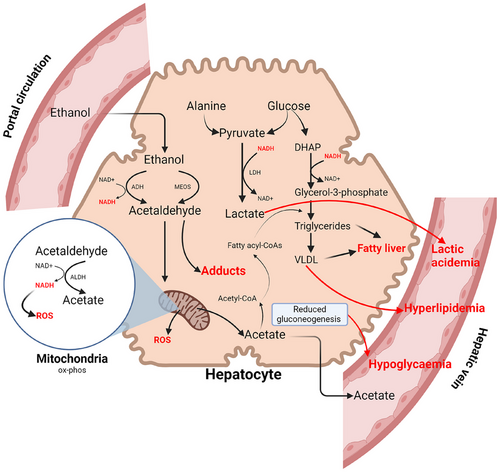
3 EPIDEMIOLOGICAL EVIDENCE
3.1 Global distribution of alcohol-related oesophageal cancers
The occurrence of the two main oesophageal cancer types, ESCC and EAC, can differ greatly according to geographical variation.19 In high-incidence areas such as Eastern Asia, Southern and Eastern Africa, more than 90% of ECs are ESCCs.19, 6 The primary risk factors in these areas are not yet well understood but an unbalanced diet and high-temperature drinks could be the contributing factors.19 China alone contributes to around half of the ES cases globally and the rate can vary widely among separate locations within China.20 In contrast, EAC is more common in low-risk areas consisting of Central America, Western and Northern Africa.19, 6 The rate in these areas is related to factors such as higher body mass index (BMI) and Barrett's Oesophagus.19 Within the US, the incidence of EC is the highest among Caucasians.20 Mortality rates for male are highest in high-risk regions and lowest in low-risk regions.6 Among all cancers attributable to alcohol consumption, oesophageal cancer (ES) has the highest proportion (26%), as compared with cancers in other organs such as the liver (21%), breast (13%) and colon (12%).21 As a well-established risk factor for ESCC, alcohol consumption has varying effects on different populations.20 In the US, alcohol is attributable to 72.4% of ESCC cases whereas in China, alcohol is only involved in 10.9% of ESCC cases.20
3.2 Impact of alcohol consumption patterns on oesophageal cancer risk
The average amount of alcohol consumption is a more relevant predictor because the duration of alcohol drinking habit has not demonstrated an association with the risk of EC.22 In terms of total alcohol intake, the dose of alcohol consumption has shown no effect on EAC and esophagogastric junction adenocarcinoma (EGJAC).23 However, alcohol dose was associated nonlinearly with the risk of ESCC.23 Compared to never drinkers, former and current drinkers respectively had a 4.3 and 2.1-fold risk increase.22 Observed together with smoking status, the risk of ESCC among people who never smoke was elevated by 3% per 10 g/week of alcohol intake.23 Among current smokers, the risk increased by 8%.23 On the other hand, there was a 6% decrease in the risk of ESCC per 10 g/week among ex-smokers.23 The highest risks for ESCC occurred among current drinkers who consume more than 170 g of alcohol per week.23 Above the value of 170 g/week, the risk of ESCC increased by 2% for every additional 10 g of alcohol intake per week.23
Looking into specific beverage consumption, there is a significant linear relationship between all levels of beer intake and the risk of ESCC.23 For each additional 10 g of beer intake per week, the risk increased by 5%.23 For wine, spirits, and port/sherry, a nonlinear relationship was observed. Low and moderate intakes of these beverages (less than 10 g for sherry or liqueur or not more than 90 g of wine per week) reduced the risks for EAC, EGJAC and ESCC.23 However, the risks of ESCC increased significantly with elevated levels of wine and spirits intake.23 Moderate consumption of wine, spirits, and sherry appears to reduce oesophageal cancer risks, but excessive intake of these beverages, especially beer, is linked to a notable rise in the likelihood of developing ESCC.
4 PATHOPHYSIOLOGICAL PATHWAYS
4.1 Effects of alcohol on oesophageal mucosal integrity
The mucosal layer lining the oesophagus lumen consists of 3 layers: non-keratinized stratified squamous epithelium, followed by lamina propria and muscularis mucosa.24 Factors impacting the mucosal integrity include the expansion of intercellular spaces and disrupting of the epithelial barrier.25 Consumption of alcohol increases the risk of squamous cell carcinoma targeting the mucosal lining. As shown in Figure 2, the metabolism of alcohol in the liver is catalysed by the enzymes ADH, CYP2E1, and catalase, producing acetaldehyde. If accumulated, acetaldehyde is a reactive toxic compound resulting in changes in DNA methylation, potentially causing mutations in tumour suppressor genes and oncogenes.21 Acetaldehyde dehydrogenase 2 (ALDH2) then converts acetaldehyde into acetate.26
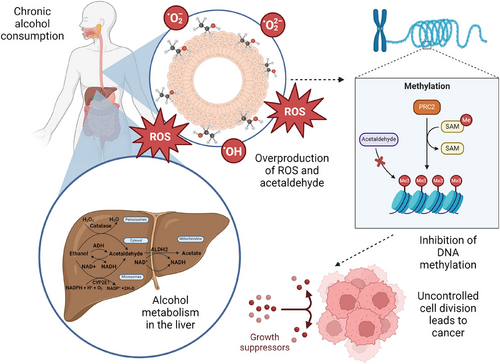
Due to the accumulation of acetaldehyde, lipid membrane peroxidation is reduced as a result of a decrease in reactive oxygen metabolites. There is a presence of direct toxic effects of alcohol due to the exposure of oesophageal mucosal lining to acetaldehyde. Around 40−50% of East Asians experience heightened exposure to acetaldehyde due to a decrease in ALDH2 activity, consequently making them susceptible to the carcinogenic effects of alcohol.27
4.2 Alcohol-induced gastroesophageal reflux disease and its contribution to carcinogenesis
Consumption of alcohol increases the risk of ESCC targeting the mucosal lining due to a condition known as gastroesophageal reflux disease (GERD). GERD causes inflammation of the oesophageal due to the acidic backflow of gastric contents into the oesophageal lumen.28 Risk factors of GERD include consumption of alcohol as it weakens the contraction of the lower oesophageal sphincter and affects the motility of the digestive tract as it delays the rate of stomach emptying, consequently increasing the chances of backflow of acid contents.29 A study by Tan et al.30 has proved that there is a positive correlation between GERD and EAC. Exposure to acidic contents causes an increase in the production of pro-inflammatory cytokines like interleukins-1, 6, 8, and tumour necrosis factor-alpha by oesophageal epithelium cells.31 According to a study by Morozov et al.,32 expression of IL-1β, IL-18, TNFA, TLR4, CD68, and β−2 microglobulin increases when exposed to acidic contents. However, the exposure decreases the expression of IL-18, TNF-α, GATA3, TLR4, and CD68.
The dysregulation of cytokines results in disruption to the apical junction and downregulation of proteins in tight junctions namely claudin-1, claudin-2, claudin-4, zonulin, occludin, and cell adhesion proteins. Coupled with the dilation of intercellular spaces of oesophageal epithelial cells,33 oesophageal tight junction integrity is compromised as toxic luminal substances are able to penetrate the mucosal layer.34 As mucosal integrity is compromised, the accumulation of ROS will increase cell proliferation. Pro-survival pathways such as phosphatidylinositol 3-kinase, MAPK1/2, and NF-kB will be upregulated due to ROS. Consequently, apoptosis is inhibited while immune evasion is favoured due to the effects of upregulation in pro-inflammatory pathways and disruption of oesophageal epithelium,35 as expressed in Figure 3. Due to the harmful effects of GERD, there is a higher chance for dysplasia and neoplasia to occur in the mucosal layer leading to BE which is a risk factor for EAC.36 Every year, it is estimated that BE patients who develop EAC are approximately 0.1−0.5%. Nevertheless, 40% of BE patients with high-grade (HG) dysplasia, develop into EAC 40% per year.37
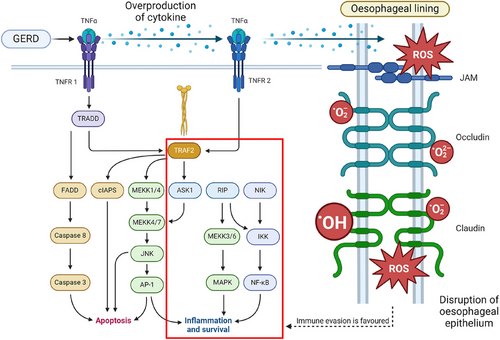
5 GENETIC SUSCEPTIBILITY AND ALCOHOL-INDUCED OESOPHAGEAL CANCERS
5.1 Genetic variants and susceptibility to alcohol-related oesophageal cancers
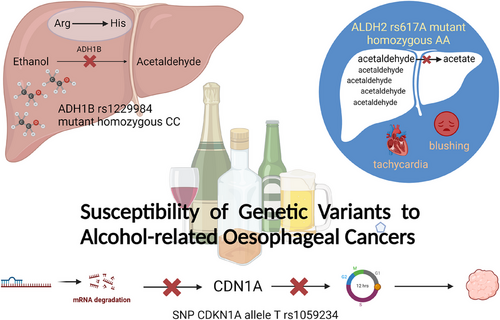
The genetic variant of ADH1B rs1229984 is associated with moderate evidence of an increased risk of ESCC. Mapped to 4q23, it encodes alcohol dehydrogenase (ADH1B) proteins that catalyse the conversion of ethanol into acetaldehyde in the liver. A non-synonymous SNP in exon 3 of ADH1B encoding for lysine-504, the rs1229984, is prone to T-to-C mutations, causing Arg-to-His mutations. As a result, the alcohol dehydrogenases translated, particularly by the mutant homozygous CC genotype, have very little activity in metabolising ethanol into acetaldehyde.38 Besides, in 2022, a prospective study of 9339 Chinese men, with 11 years of follow-up, associates the rs1229984 AA genotype with a decreased risk of alcohol-related cancers compared to the GG genotype.39
On the other hand, the ALDH2 rs671A allele significantly decreases the risk of ESCC by 40% because of its failure in acetaldehyde metabolism. Located on chromosome 12q24.2, ALDH2 encodes aldehyde dehydrogenase 2 protein, which catalyses the oxidation of acetaldehyde to acetate in the liver. Wild homozygous genotype GG of ALDH2 rs671 encodes normal aldehyde dehydrogenases, while heterozygous GA genotype encodes enzymes with reduced activity. Unfortunately, individuals with mutant homozygous AA genotype have completely inactive aldehyde dehydrogenases, causing them to experience tachycardia, blushing, and other unpleasant symptoms after drinking due to the massive accumulation of acetaldehyde in their bodies.38 It is found that IL-4 rs2243263 G > C polymorphism increases the risk of ESCC in obese and overweight individuals. Meanwhile, IL-4 rs2070874 T > C polymorphism is identified as a protective factor, with IL-4 rs2070874 CC genotype significantly lowering the risk for ESCC development compared with TT and TC genotypes.40 This is consistent with another meta-analysis which links the IL-4 rs2070874 C allele with reduced susceptibility to gastrointestinal cancers.41 A possible explanation is that IL-4 rs2070874 allele C increases plasma IL-4 level,42 which has an anti-inflammatory effect and reduces the risk of developing ESCC.
SNP CDKN1A rs1059234 increases the risk of ESCC.43 CDKN1A gene, located in chromosome number 6, encodes for cyclin-dependent kinase inhibitor-1 (CDN1A), a downstream signalling protein which is regulated by the P53 tumour suppressor protein.44 When the DNA of a cell is damaged, the CDN1A level increases, causing cell cycle arrest between the G1 and S phases.45 Allele T of rs1059234 produces an unstable, rapidly hydrolysed mRNA, resulting in lesser amounts of CDN1A produced via translation.46, 47 As a result, uncontrolled cellular growth and carcinogenesis are more likely to occur48, 49 (Figure 4).
5.2 Epigenetic modifications in the context of chronic alcohol consumption
Epigenetics can be broadly defined as changes to genetic expression without any change in the DNA sequence.50 Three major epigenetic modifications are identified in the context of chronic alcohol consumption, namely DNA methylation, histone modifications, and RNA-based mechanisms.
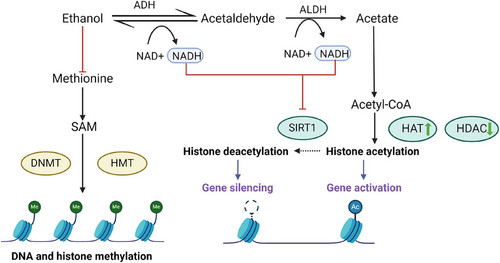
Chronic alcohol consumers may have their DNA methylation patterns altered in two ways that can affect the risk of cancer development. Firstly, the ROS and acetaldehyde produced can damage DNA and cause global hypomethylation, which, in turn, activates oncogenes while silencing the tumour suppressor genes. Secondly, gene-specific hypermethylation can be induced by alcohol consumption, inactivating the genes regulating DNA repair, cell cycle, and apoptosis.
Besides, long-term alcoholism can also cause histone modifications, namely histone acetylation and methylation. Chronic alcohol consumption increases the acetylation of histones associated with oncogenes, activating them. Meanwhile, tumour suppressor genes are silenced through reduced histone methylation.
Chronic alcohol consumption can also alter RNA-based mechanisms, particularly the expression of microRNAs (miRNAs) and long noncoding RNAs (lncRNAs). miRNAs are small, non-coding RNAs that change the expression of oncogenes or tumour suppressor genes, whereas lncRNAs can regulate gene expression by acting as oncogenes or tumour suppressor genes, both of which can contribute to cancer development.
These alcohol-induced epigenetic changes can be detected in blood as epigenetic markers, thus offering valuable diagnostic, therapeutic, and prognostic insights into various alcohol-related problems.51 Acute exposure of humans to ethanol generally increases all 11 histone deacetylases (HDACs) except HDAC9 and HDAC10 in blood,52 suggesting tissue-specific regulation of HDACs by ethanol. The complex interaction of histone acetylases (HATs) and HDACs affects histone acetylation. However, in the context of chronic ethanol exposure and withdrawal, histone acetylation is generally reduced through the downregulation of HATs in the amygdala and upregulation of HDACs, consequently decreasing gene expression that regulates neuroplasticity or neurotransmission.53 For example, increased anxiety-like phenotype, corresponding to a decrease in H3K9 and H4K8 acetylation, and CBP and NPY expressions, is observed during withdrawals of chronic ethanol exposure.54 Following chronic ethanol consumption and withdrawal, numerous histone methylation genes are dysregulated.53 In conclusion, long-term alcohol exposure and withdrawal activate the amygdala and the bed nucleus of the stria terminalis, also known as the negative affect centres, causing a decrease in histone acetylation, an increase in repressive histone methylation, and a repressed gene expression, which result in increased dysphoric behaviours such as anxiety53 (Figure 5).
6 BIOMARKERS AND EARLY DETECTION
6.1 Identification of biomarkers for alcohol-related oesophageal cancers
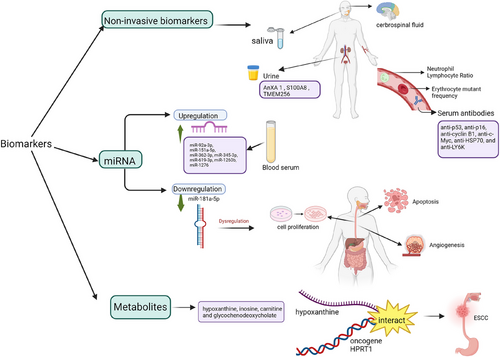
In the early stages of oesophageal cancer, it is often asymptomatic, resulting in a 5-year survival rate of less than 20%.55 Hence, to ensure detection of cancer is highly specific for early oesophageal cancer diagnosis, non-invasive biomarkers, miRNA biomarkers and metabolites biomarkers are used (Figure 6).
6.2 Non-invasive biomarkers
Body fluids such as urine, saliva, and cerebrospinal fluid can be collected to track the location of the tumour [1]. For diagnosis of Stage 1 ESCC, urinary biomarkers such as ANXA1, S100A8, and TMEM256 can classify ESCC, and proteins ANXA1, S100A8, SOD3, and TMEM256 are selected. To differentiate between EAC and BE, neutrophil-lymphocyte ratio, erythrocyte mutant frequency, and serum antibodies (anti-p53, anti-p16, anti-cyclin B1, anti-c-Myc, anti-HSP70, and anti-LY6K) can be used.56
6.3 miRNA biomarkers
During the preliminary stages of EAC, miR-92a-3p, miR-151a-5p, miR-362-3p, miR-345-3p, miR-619-3p, miR-1260b, and miR-1276 in blood serum increases. Conversely, there is a decrease in miR-381-3p, miR-502-3p, and miR-3615.57 Another study conducted by Lin et al.58 in relation to miRNAs associated with EC shows the level of miR-34a-5p increased, but the levels of miR-148a-3p and miR-181a-5p decreased in EC patients when in comparison to healthy subjects. As there is no significant difference between factors such as sex and age, miR-181a-5p can be used to diagnose the early stages of EC. Results proved that there is a decrease in the expression of miR-181a-5p in plasma compared to normal subjects. Downregulation of miR-181a-5p causes dysregulation of angiogenesis, cell proliferation and inhibition of apoptosis, leading to cancer development.59, 60 miRNAs can be used to determine types of tumours and the TNM stage. Nonetheless, mature miRNAs are difficult to find as they are present in small numbers. Besides, while detecting miRNAs, they are susceptible to changes in homologous sequence as the structure of the mature miRNA is short, only 21 to 25 nucleotides long.58
6.4 Metabolites biomarker
Based on 653 serum samples that were collected in a study by Sun et al.,61 metabolites such as hypoxanthine, inosine, carnitine, and glycochenodeoxycholate can be used as biomarkers for early diagnosis of ESCC. In purine metabolism, an increase in levels of hypoxanthine/xanthine-HPRT1 is related to pathological aspects of ESCC.62 This can be used as a biomarker as there is a high need for energy in tumour cells for replication of DNA by obtaining nucleotides via purine metabolism. Not to mention that hypoxanthine synergises with oncogene HPRT1, which contributes to ESCC development.61
6.5 Screening approaches for early diagnosis and management
Only one out of eight oesophageal cancers are identified at an early stage (T1).63 The most usual form of screening for oesophageal cancer is oesophageal endoscopy with biopsy. Endoscopic screening allows early detection of lesions in patients who have a high risk of developing ESCC.64 However, it is not financially accessible in countries where the disease is most prevalent.65 Hence, 40% of early ESCCs are left undetected even in high-risk populations.66 However, it is crucial to note that detection of early stages of ESCC poses challenges as it is often overlooked due to its flat appearance, usually flat Paris type IIa, IIb, or IIc lesion. These issues can be due to disparities in healthcare access, screening accessibility, and prevention strategies across different regions, especially in low-income areas. Moreover, financial barriers, transportation issues, healthcare worker shortages, and lack of evidence-based guidelines are also factors that can lead to poorer cancer treatment outcomes. These can be determined by availability, accessibility, affordability, and acceptability that affect a person's ability to access cancer screening and treatment. To overcome this, newer endoscopic techniques such as dye spray chromoendoscopy, virtual chromoendoscopy, confocal laser endomicroscopy, and volumetric laser endomicroscopy are used.67 These technologies aid in the visualisation of abnormal mucosal lining which increases the chances of finding small lesions. Hence, detection of ESCC in an early stage can allow endoscopic resection to be performed, including endoscopic mucosal resection and endoscopic submucosal dissection, which are highly preferred as they are minimally invasive.68
7 PREVENTIVE STRATEGIES
7.1 Lifestyle modifications for reducing oesophageal cancer risk in alcohol consumers
A major focus is posed on highlighting the intervention strategies for oesophageal cancer, particularly for those with precancerous conditions or high-risk profiles, with the final aim of detecting these lesions as early as possible to prevent further complications. These approaches include lifestyle modifications, pharmacological intervention, and enhanced diagnostic and screening processes to improve cancer outcomes and reduce cancer risk in susceptible populations.
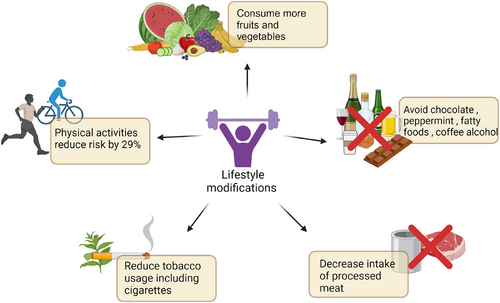
In 2018, the WCRF/AICR Continuous Update Project Expert Report states that body mass index, waist circumference, and waist/hip ratio are risk factors for EAC. However, there is no evidence that dietary fibre intake decreases the risk of ESCC.69 Torrelation between diet and cancer risk is contradictory based on the Expert Report, the World Cancer Research Fund/American Institute for Cancer Research states that increasing intakes of vegetables and fruits, while decreasing intakes of processed meat contributes to lower risks of ESCC and EAC.70 Consumption of fruits and vegetables is extremely beneficial as it contains antioxidant properties which decreases DNA damage, and they contain flavones which prevents carcinogenesis.37
The American College of Gastroenterology advises patients to avoid foods such as chocolate, peppermint, fatty foods, coffee, and alcohol as they may worsen the symptoms of GERD, leading to dysplasia of oesophageal lining.71 The US National Institutes of Health-American Association of Retired Persons Diet and Health study, shows that following a Mediterranean Diet is associated with a significantly reduced risk of ESCC, but not EAC.70
As shown in Figure 7, the American College of Sports Medicine demonstrates a positive correlation between physical activities and the prevention of oesophageal cancer.72 A meta-analysis conducted by Singh et al.73 shows those who are involved in physical exercise have a 29% decrease in risk of developing oesophageal cancer compared with subjects who do not. Exercise has an effect on visceral fat reduction and C-peptide, and improves sensitivity to insulin. Other lifestyle modifications include reducing tobacco usage which is one of the risk factors. Carcinogenic compounds in cigarette smoke form DNA adducts which may cause FHIT gene mutation. FHIT gene mutation has a strong correlation to the development of oesophageal cancer.27
7.2 Potential role of pharmacological interventions in high-risk populations
In treating precancerous stages, there are no currently available drugs but research is underway. A study conducted by Laura et al. on rats shows that lyophilised black raspberries prevent oesophageal tumour formation caused by N-nitrosomethylbenzylamine.74
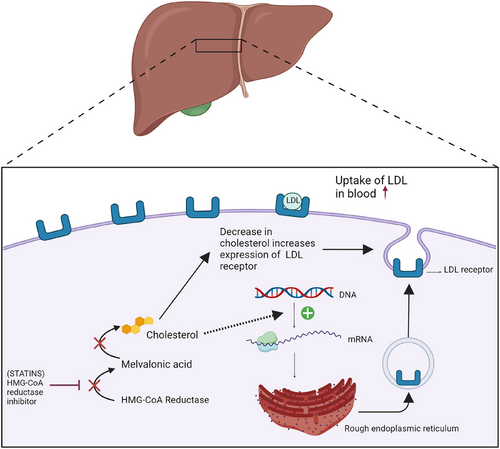
Pharmacological interventions are necessary to prevent the advancement of the disease among high-risk individuals, especially in BE patients who are at risk of developing EAC.37 Patients with a history of BE who took statins have a 43% decreased risk of developing oesophageal cancer. According to a meta-analysis conducted by Beales et al. (2013), 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, also known as statins, have anti-cancer properties such as regulating the inhibition of proliferation and enhancing apoptosis.75, 76 Cancer cells that are metabolically active require high levels of cholesterol to synthesise plasma membrane. Thus, statins that reduce levels of low-density lipoprotein cholesterol by enhancing the expression of LDL receptors, inhibit the development of cancer.77 Besides, statins inhibit the activation of the proteasome pathway which downregulates the breakdown of cyclin-dependent kinase inhibitors p21 and p27. Thus, the cell cycle can be inhibited.37 As for the dosage of statin, a higher dose (simvastatin ≥40 mg or equivalent) has proven to reap more advantages for both types of oesophageal cancer, in comparison to a lower dosage (simvastatin < 40 mg per day).75 However, a study by Marabotto et al. (2021) demonstrates that statin is not recommended for chemopreventative treatment for cancers,37 as shown in Figure 8. Proton pump inhibitors (PPIs) can be administered as a treatment for BE patients. PPIs act as irreversible inhibitors of H+/K+ ATPase enzymes which reduce the duration of acidic oesophageal exposure. The dosage for PPI is once daily as stated by clinical guidelines. A study proves that usage of PPI has a 75% reduction in the risk of neoplastic progression in patients with BE.37
8 TREATMENT MODALITIES AND PROGNOSIS
8.1 Current therapeutic approaches for alcohol-associated oesophageal cancers
As shown in Figure 9, esophagectomy, or surgical resection of part or all of the oesophagus, had been the traditional curative treatment method in oesophageal cancer for years. But recently, endoscopic en bloc resection, preferably via endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), has been established as the gold standard in early ESCC, staged as T1N0M0, due to the reduced morbidity and mortality rates. EMR is a simpler and faster technique to use, but it is limited by its inability to resect large lesions en bloc. There is also a high rate of recurrence if EMR is performed on large lesions. ESD was developed as an alternative to EMR, facilitating en bloc resection and histopathologic assessment. Despite being a more technically challenging procedure, ESD has en bloc resection rates of 83–100%, complete resection rates of 78–100%, and local recurrence rates of 0–2.6% in superficial ESCCs. If the deep resection margin is infiltrated by tumour cells or the patient shows risk factors for lymph node metastases such as lymphovascular invasion, low differentiation grade, ulceration and large tumour size, then further resective surgery with lymphadenectomy, a surgery to dissect and remove lymph nodes, should be recommended.78
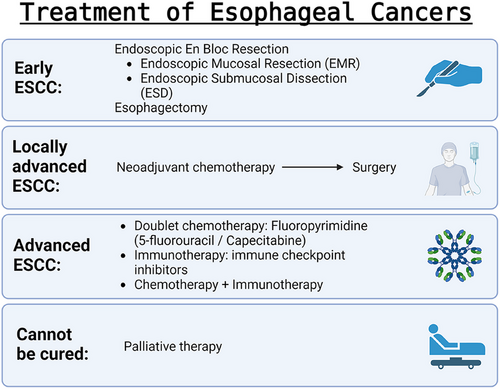
For locally advanced ESCCs, staged T2-4 or N1-3 with M0, the main treatment accepted globally is neoadjuvant chemoradiotherapy, independent of molecular markers, followed by surgery. This is because most ESCCs are sensitive to chemoradiation, and having chemotherapy before surgery improves rates of complete tumour regression and local tumour control.9, 79 ESCC is staged as advanced when it has become metastatic, unresectable, or cannot be treated with definitive chemoradiotherapy. First-line systemic therapy comprises either doublet chemotherapy based on fluoropyrimidine (5-fluorouracil or capecitabine) with either oxaliplatin or cisplatin,80, 81 immune checkpoint inhibitors (immunotherapy), or a combination of both. Possible combinations include pembrolizumab + chemotherapy, nivolumab + chemotherapy, and nivolumab + ipilimumab.78, 82 Over 50% of the patients are diagnosed with oesophageal cancer that cannot be cured. They should be offered palliative therapy,83 based on their symptoms, preference, and performance status.9
8.2 Prognostic factors and survival rates in alcohol-related oesophageal cancers
According to the National Cancer Institute of the United States government, the prognostic factor is a characteristic of a patient, condition, or situation, which can be used to estimate the likelihood of recovering from a disease or the likelihood of the disease recurring. The most important prognostic factor in alcohol-related oesophageal cancers is the stage of cancer, particularly the presence (stage IV) or absence (stages I–III, localised or locally advanced) of distant metastases.84 Sadly, most patients are diagnosed with oesophageal cancer at an advanced stage with limited treatment options, and thus prognosis and survival rate remain poor even in high-income countries,6 with less than 20% of patients managing to survive for more than 5 years after diagnosis, causing oesophageal cancer to be one of the most lethal cancers globally.85
The 5-year survival rates for both ESCC and EAC combined in the US range from 46.4% for localised tumours to 5.2% for stage IV patients. Stage IV survival rates do not differ significantly between ESCC and EAC, with 5-year overall survival ranging from 5.0% to 7.5% for ESCC and 4.3–5.8% for EAC.84 While the US National Cancer Institute estimates the 5-year relative survival rate of oesophageal cancer to be 21.6%. Between 2014 and 2020, 18% of oesophageal cancer incident cases from the United States were localized (confined to the primary site), 32% were regional (spread to regional lymph nodes), 39% were distant (cancer has metastasised), and the remaining 11% were unstaged. The 5-year relative survival rate for localised oesophageal cancer is 48.1%, regional 28.1%, distant 5.3%, and for those unstaged, 15.9%. According to Cancer Research UK, between 2013 to 2017, 12% of patients in England have survived oesophageal cancer for 10 years or more.
9 PUBLIC HEALTH IMPLICATIONS AND POLICY RECOMMENDATIONS
9.1 Public health interventions for alcohol-related cancer prevention
Since there is a relationship between alcohol dose and cancer risk, implementing policies to limit the consumption of alcohol will be an effective way to reduce the occurrence of alcohol-related cancers.86 As expressed in Figure 10, the policies can focus on altering the availability, accessibility, affordability, and advertising of alcohol. It is found that a 10% increase in alcohol price causes a 5% reduction in alcohol consumption.86 Taxes based on the alcohol content of beverages with inflation considered have been proven to reduce alcohol consumption and its related harm in a country.86 This strategy applies to all groups of drinkers and works at the population level.86 Furthermore, a growing number of evidence shows a strong association between the density of alcohol outlets and the rate of alcohol-related diseases.86 Hence, making adjustments to liquor licensing requirements and legal drinking age could potentially reduce alcohol availability and accessibility. Moreover, some studies suggest high levels of alcohol marketing exposure caused young people to continue to drink more heavily as they become older.86 Therefore, restricting alcohol advertising and regulating media promotion may be effective in lowering alcohol consumption. Besides legal interventions, drink-driving campaigns and occupational health strategies in the workplace are also some popular ways to reduce alcohol consumption.
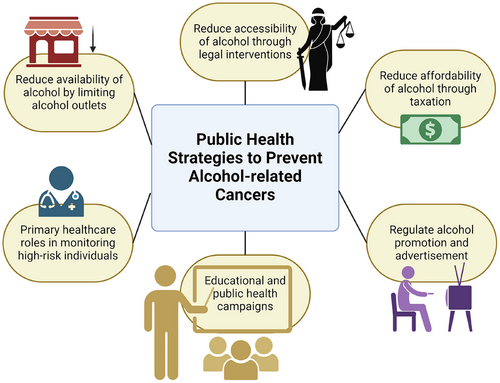
Another strategy to prevent alcohol-related cancer is by identifying the high-risk population through primary health care.87 General practitioners (GPs) play an important role in collecting information and monitoring patients’ levels of drinking.87 This is done by asking patients questions, keeping diaries, and doing physical examinations.87 By doing these, they can make opportunistic interventions to promote healthier lifestyles among their patients.87 This is especially important for heavy drinkers because they see their GPs twice as frequently as light drinkers, allowing the GPs to target those at risk more easily.87 Some studies have shown that 5 to 10 min of brief advice from a GP combined with a leaflet resulted in a 25–35% reduction in alcohol consumption 6 months to 1 year later.87 A 45% reduction was seen among excessive drinkers.87
The disparities in terms of healthcare access, screening and prevention across different regions need to be addressed. In low-income areas, patients have limited access to high-quality medical services. They are less likely to participate in cancer screenings due to factors such as lack of awareness and poor health literacy. They may also have difficulties accessing services like endoscopy, which is helpful in detecting early-stage oesophageal cancer. Moreover, there are often delays in receiving timely treatments after diagnosis. For instance, patients from rural areas may face challenges accessing esophagectomy surgeries, which are only available in specific medical centres. Some patients from disadvantaged backgrounds may also rely on public insurance programs, which may not fully cover the cost of advanced treatments such as chemoradiation. Furthermore, individuals from low socioeconomic backgrounds are often exposed to poor lifestyle factors, such as excessive alcohol consumption, smoking, and poor dietary habits. These factors, combined with other social determinants of health such as unemployment and limited access to recreational activities, increase the risk of oesophageal cancer.
9.2 Regulatory measures and awareness programs for mitigating oesophageal cancer risk
For EAC, the population risks can be possibly reduced through public health campaigns that aim to discourage smoking habits and encourage weight loss in overweight people.88 There are ongoing global initiatives targeting obesity as well as prevention programs related to lifestyle modifications.89 Some studies also suggest control of acid reflux as a beneficial method to reduce EAC risk.88 For ESCC, the primary prevention strategies can vary from population to population.88 For instance, abstinence from alcohol consumption and smoking in Western nations is critical because it leads to a 75 to 90% reduction in ESCC incidence.88 In China, high-risk populations are targeted with large-scale interventions that focus on certain micronutrients and food groups.88 The National Cohort of Oesophageal Cancer-Prospective Cohort Study of Oesophageal Cancer and Precancerous Lesions Based on High-Risk Populations has also been developed to identify risk factors and refine screening guidelines for EC prevention.89 The study demonstrates the effectiveness of upper GI screening in decreasing the incidence of EC and mortality within a high-risk population.89 In Iran, the primary prevention is done by lowering opium and scalding tea consumption.88 Generally, harmful cooking practices should be avoided in all populations.88 At the same time, programs that encourage proper oral hygiene and consumption of fresh fruits and vegetables proved beneficial.88 Since dysphagia is the most common symptom of EC, some studies suggest raising public awareness about the symptoms of dysphagia can be key to allowing early detection and diagnosis of EC.90
10 CONCLUSION
10.1 Summary of key findings and implications
In a nutshell, despite recent advancements in treatment modalities, oesophageal cancer remains a threat to our society. 90% of the cases worldwide are contributed by ESCC, and the major risk factor for ESCC is chronic alcohol consumption. Ethanol is converted by alcohol dehydrogenase to acetaldehyde, which is then metabolised by aldehyde dehydrogenase to form acetate in the liver. Acetaldehyde is carcinogenic, either by damaging the DNA, inhibiting DNA repair, or reacting with DNA bases to form DNA adducts. The alternative alcohol metabolising pathway involving the CYP2E1 enzyme generates large amounts of ROS such as hydroxyl radicals and superoxide anions, inducing oxidative stress which consequently drives mutations and increases the risk of carcinogenesis. Additionally, alcohol metabolism can interfere with the metabolism of various nutrients, causing nutritional deficiencies and fatty liver. Meanwhile, consumption of alcohol weakens the lower oesophageal sphincter, resulting in an increased risk for GERD and BE. GERD and BE are commonly associated with the development of EAC via dysregulation of cytokines which compromises the oesophageal mucosal integrity, causing ROS to accumulate. Numerous genetic variants have been found to affect susceptibility to oesophageal cancers: ADH1B rs1229984 increases the risk of ESCC and ALDH2 rs671A allele decreases the risk of ESCC, to name a few. Three major epigenetic modifications are identified in the context of chronic alcohol consumption, namely DNA methylation, histone modifications, and RNA-based mechanisms. The prognosis of alcohol-related oesophageal cancer is poor and can be improved via early diagnosis, made possible through biomarkers (non-invasive biomarkers, miRNA biomarkers, and metabolites biomarkers) and newer endoscopic techniques (dye spray chromoendoscopy, virtual chromoendoscopy, confocal laser endomicroscopy, and volumetric laser endomicroscopy). Therapeutic regimens depend on the stage and grade, and include endoscopic resections (EMR and ESD), surgery (esophagectomy), chemotherapy, radiotherapy, palliative, and targeted drugs and immunotherapy which is a promising field requiring further investigations. For high-risk alcohol consumers, lifestyle modifications such as diet, physical activity, and reduced consumption of alcohol and tobacco can help in reducing the risk of developing EC. Pharmacological interventions (statins and proton pump inhibitors) may help patients with BE to decrease their risk too. Lastly, the government should work to decrease the availability, accessibility, and affordability of alcohol, regulate its advertising, and organise more health awareness campaigns that target various risk factors of EC.
10.2 Future research directions and emerging perspectives
Almost 50% of locally advanced oesophageal cancer patients who underwent some form of chemoradiation followed by surgery have a recurrence of cancer. The latest research for treatment options for EC includes immune checkpoint inhibitors to reduce the mortality rate. Examples of the latest immunotherapies include anti-PD1 agents (nivolumab, pembrolizumab, camrelizumab, tislelizumab, sintilimab, and toripalimab), anti-PD-L1 agents (atezolizumab and avelumab), and anti-CTLA4 agents (ipilimumab and tremelimumab).35 Emerging immunotherapies, such as immune checkpoint inhibitors targeting PD-1, PD-L1, and CTLA-4, show promise in reducing recurrence and improving survival rates for patients with locally advanced oesophageal cancer. ESCC progresses from the tumour's microenvironment which contains cancer-associated fibroblasts (CAFs) In CAF cells, there is a high expression of Periostin (POSTN).91 A study by Ishibashi et al.92 proved that the proliferation of cells is enhanced in presence of POSTN as Erk pathway via αvβ3 or αvβ5 is switched on. Hence, this may be a frontier pharmacological target to treat ESCC.91 Future initiatives include collaboration between countries for global research, investing in AI diagnostic measures, and precision medicine to tailor to individual risk factors and genetic profiles.93
AUTHOR CONTRIBUTIONS
Chen Huai Yi, Por Chia Rou, Hong Yong Kai, and Vetriselvan Subramaniyan conceived and designed the structure of the review. Chen Huai Yi, Por Chia Rou, and Hong Yong Kai conducted literature research and drafted the entire manuscript. Eason Qi Zheng Kong and Vetriselvan Subramaniyan edited the manuscript. Chen Huai Yi, Por Chia Rou, Hong Yong Kai, and Eason Qi Zheng Kong contributed to the key parts of the text associated with it. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
The authors express our sincere gratitude to Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia for providing the necessary support to successfully complete this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Ethical approval was not required for this review study as it did not involve primary data collection or human or animal subjects. The entire study was supported by the Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia.




