Endothelial cell senescence contributes to pathological retinal angiogenesis
Abstract
Background
Pathological retinal neovascularization is marked by microvascular lesions manifested initially as endothelial cell dysfunction and metabolic disturbances. However, the regulatory mechanism guiding retinal vascular endothelial cell function remian controversial.
Main body
Previous studies have demonstarted that high glucose or oxidative stress can induce premature senescence in endothelial cells, triggering inflammatory responses within the vascular system and promoting the secretion of pro-inflammatory factors, ultimately leading to pathological angiogenesis. Endothelial cell senescence has thus become a key target for anti-angiogenic therapies.
Conclusion
This review delves into current research on the mechanisms driving senescence-induced retinal angiogenesis and highlights potential target protein pathways, aiming to provide insights for future investigations.
1 INTRODUCTION
Pathological neovascularization in the retina is a critical factor leading to vision loss in patients with ocular fundus diseases, particularly diabetic retinopathy (DR), retinopathy of prematurity (ROP) and wet age-related macular degeneration (wAMD).1, 2 Abnormal angiogenesis is a pathological response to retinal cell damage or metabolic disorder. Compared with normal blood vessels, pathological neovascularization is structurally abnormal, has increased permeability, and is prone to leakage and bleeding, aggravating retinal injury and causing visual impairment. Current treatments include laser photocoagulation and injection of anti-vascular endothelial growth factor (anti-VEGF) drugs to inhibit the growth of abnormal blood vessels and reduce vascular leakage.3, 4 However, the risk of invasive procedures and drug sensitivity differences make the treatment effect unsatisfactory. Consequently, there is a pressing need to identify new therapeutic targets to effectively inhibit pathological angiogenesis.
In the vascular system, endothelial cells are pivotal in maintaining vascular structure and function. The senescence of endothelial cells is accompanied by decreased proliferation, reduced apoptosis, heightened oxidative stress and activation of inflammatory responses. These changes impair vascular repair and promote pathological angiogenesis.5, 6 Therefore, the accumulation of senescent endothelial cells contributes to vascular dysfunction and the progression of tumour and ageing-related cardiovascular diseases,7 including atherosclerosis, heart failure and pulmonary hypertension.8 Research indicated that in the vascular-aging disease model, inhibition of endothelial cell senescence promotes new vessel formation, prevents cardiac fibrosis and improves diabetic cardiomyopathy.9 However, in mouse models of retinal microvascular dysfunction, senescent endothelial cells facilitate the release of pro-angiogenic factors and induce pathological neovascularization, while inhibition of senescent cells allows for regrowth of normal blood vessels.10 These findings demonstrated that age-independent features of neovascularization disease also trigger cellular senescence. In general, endothelial cell senescence is emerging as a critical target for anti-angiogenic therapy. However, the detailed mechanisms of endothelial senescence are not fully illustrated. This review aims to elucidate the specific role of endothelial cell senescence in retinal neovascular diseases and discuss potential therapeutic strategies.
2 THE MECHANISM OF ENDOTHELIAL CELL SENESCENCE
Endothelial cells are an essential component of the vascular system, playing key roles in blood vessel formation, blood flow maintenance, blood pressure regulation and the selective penetration of substances. Endothelial cell senescence is caused by various mechanisms, including oxidative stress, inflammation, telomere dysfunction, accumulation of DNA damage, ultraviolet radiation and autophagy dysfunction.11, 12 There are three main types of cellular senescence: replicative senescence (RS), stress-induced premature senescence (SIPS), and developmentally programmed senescence (DPS).13 RS depends on telomere erosion or dysfunction, whereas SIPS is telomere-independent.14 Nonetheless, the initiation and induction of RS and SIPS seem to depend on the same tumour suppressor pathways, p53-p21CIP1 and p16Ink4a-retinoblastoma protein (pRB), leading to cell cycle arrest.13 DPS is a physiological process coordinated by developmental signals to promote tissue regeneration and repair.15 Senescent endothelial cells show reduced proliferation, apoptosis and migration capabilities, in a sense, cellular senescence serves as a protective mechanism to halt the uncontrolled proliferation of damaged cells.16 In retinal microvascular lesions, senescence is primarily driven by glycemia or oxygen-induced premature senescence.17 Under pathological conditions, endothelial cells are exposed to the bloodstream and stress such as inflammation, oxidative stress, high glucose, and high lipid levels, all of which induce senescence.18 Senescent endothelial cells are characterized by the senescence-related secretory phenotype (SASP), releasing a host of inflammatory chemokines, cytokines and growth factors that lead to vascular dysfunction, including reduced vasodilation function, impaired angiogenesis and increased vascular permeability.18, 19 Overall, senescence is the cellular response to injury and characterized by the massive production of cytokines and other secreted factors, along with the recruitment of inflammatory cells, leading to tissue remodelling.20 Mounting evidence indicates that vascular endothelial senescence is the key to endothelial dysfunction and is intimately involved in vascular repair and remodelling. In the retinal vasculature, endothelial cell senescence-induced metabolic dysfunction, chronic inflammation and expression of SASP factors promote pathological vessel formation21, 22 (Figure 1).
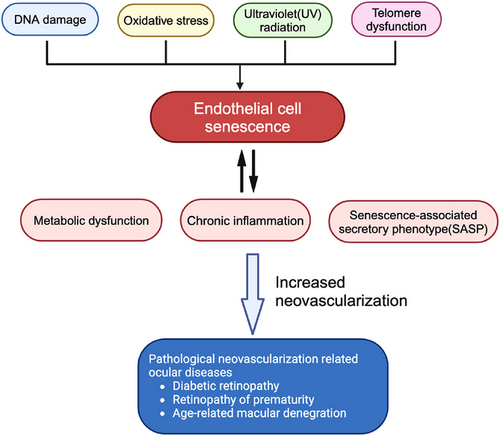
3 ENDOTHELIAL CELL SENESCENCE AND RETINAL PATHOLOGICAL ANGIOGENESIS
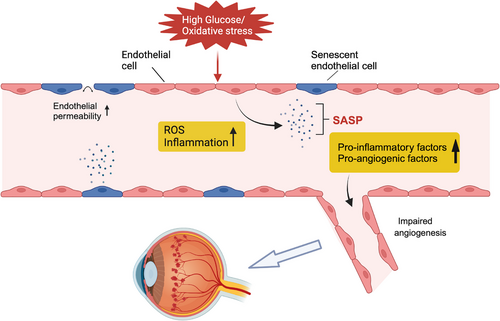
Endothelial cell senescence can impair the integrity of retinal vascular endothelium and contribute to the development of neovascularization. In diabetic mice models, a high-glucose environment induces premature senescence of endothelial cells, while oxidative stress also drives this process by producing reactive oxygen species (ROS) and causing mitochondrial dysfunction. This pathological process caused by oxidative stress is prominently observed in conditions such as oxygen-induced retinopathy (OIR) and wAMD.10, 23 The mechanism of endothelial cell senescence is more complicated in wAMD, including both age-related chronic endothelial cell senescence and stress-induced premature cell senescence.23, 24 Senescence of endothelial cells is accompanied by a weakened cellular barrier function, resulting in increased vascular wall permeability. Imperfect vascular conditions allow fluids and proteins to seep into the retina, causing or exacerbating retinal oedema. In pathological environments, the accumulation of ROS accelerates endothelial cell senescence, this activates the p53/p21 and p16Ink4a tumour suppressor pathways, which intensify the senescence state. Therefore, p16 (CDKN1A) and p21 (CDKN2A) are commonly used as markers of senescence.16 The activity of senescence-associated β-galactosidase (SA-β-gal) was significantly enhanced in the ischemic retinopathy tissue of mice. β-galactosidase is a lysosomal enzyme that is active at pH 4 in normal cells and active at pH 6 in senescent cells.25 In the neovascular area in proliferative retinopathy, the expression levels of senescence markers p16INK4A and p21 were markedly increased,10 and senescent endothelial cells are highly active and adapt to SASP, which is characterized by the secretion of various pro-inflammatory and pro-angiogenic factors, such as tumour necrosis factor-a, interleukin (IL)-6, IL-8, IL-1β, CXCL11, plasminogen activator inhibitor 1, VEGF and matrix metalloproteinases (MMPs). These factors stimulate the proliferation and migration of surrounding healthy endothelial cells, promoting the formation of new blood vessels (Figure 2).26 Moreover, in DR, the persistent chronic inflammatory state formed by SASP also contributes to pathological neovascularization. Additionally, the senescence of endothelial cells might alter the composition and function of the extracellular matrix, with increased secretion of MMPs involved in degrading the extracellular matrix, affecting cell adhesion and migration capabilities.27 The protein levels of MMP-2 and MMP-9 were increased in senescent endothelial cells. Such alterations may result in abnormal morphology and function of new vessels, creating fragile vessel walls with an increased risk of bleeding. Inhibition of MMP-2 ameliorates extracellular matrix degradation and angiogenesis28 .
4 CELL SENESCENCE-RELATED MOLECULAR PATHWAYS AND POTENTIAL THERAPEUTIC STRATEGIES
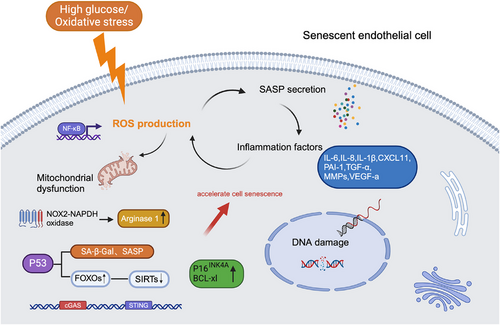
Research on endothelial cell senescence mechanisms is continuously evolving, with oxidative stress and inflammation considered key drivers of promoting cellular senescence. The activation of SASP involves triggering pro-inflammatory transcription factors like nuclear factor-κB (NF-κB), promoting ROS production, which leads to DNA damage and mitochondrial dysfunction.23, 29, 30 The damaged mitochondrial DNA further increases ROS production, thereby creating a chronic inflammatory environment through a positive feedback loop that fosters the formation of new blood vessels.31 The knockdown of the forkhead box transcription factor O1 (FOXO1) alleviated hyperglycemia-induced persistent DNA damage and cellular senescence by promoting DNA repair.32 Among the forkhead family ‘O’ group (FOXOs), FOXO1 is well characterized and plays crucial roles in cell survival, oxidative stress resistance and cell death.33 Sirtuins (SIRTs) have emerged as important mediators of endothelial cell senescence, activated through the deacetylation of FOXOs.34, 35 Previous studies have shown that the ageing process was accelerated through the depletion of SIRTs by the FOXO1 pathway.36 Further tracing upstream mechanisms, uncoupling protein 2 (UCP2) can regulate the NAD+-SIRT3 axis to relieve oxidative stress and senescence in DR.37 Another study demonstrated that inhibition of p53 can reduce the expression of SA-β-Gal and SASP in HRMECs, the specific mechanism showed that p53 can accelerate endothelial cell senescence by enhance FOXO3a ubiquitination and degeneration.38 Therefore, SIRTs and FOXOs play vital roles in the regulatory mechanism of cellular senescence. Research has shown that oxidative stress triggers NF-κB-regulated inflammatory response by activating the cGAS/STING signalling pathway.39 Elevated levels of STING and cGAS have been detected in retinal tissues from DR donor patients and diabetic mice.40 Targeting STING might be a new strategy for early prevention and treatment of DR. Additional research has found that the premature senescence of retinal endothelial cells induced by diabetes is often accompanied by the overexpression of arginase 1 (A1), and A1 gene deletion or drug inhibition can alleviate the premature senescence induced by high glucose.41 In mouse models of OIR, accumulation of senescent endothelial cells (marked by increased p16) and elevated expression of BCL-xL in neovascular tissues have been observed (Figure 3). Inhibition of cellular senescence by eliminating p16-positive cells or inhibiting BCL-xL may reduce pathological angiogenesis and promote normal angiogenesis. However, genetic removal of p16-positive cells may induce liver and perivascular fibrosis, potentially arising from the adverse effects of systemic administration of senolysis.10 From the perspective of enhanced SASP expression, studies have shown that therapeutic inhibition of SASP through intravitreal injection of metformin or interference with senescence effectors (such as semaphorin 3A) can reduce the formation of pathological neovascularization in the retina in vivo.42 Regarding the pathogenesis of wAMD, oxidative stress-induced cell senescence including endothelial senescence (retinal vascular endothelial cells and choroidal endothelial cells) and retinal pigment epithelium (RPE) cell senescence, RPE cell dysfunction plays a more important role in the development of wAMD. RPE cells are essential for the maintenance of retinal homeostasis through the blood-retinal barrier (BRB), the functional decline of senescent RPE cells disrupts the BRB and causes photoreceptor dysfunction.23 Up-regulation of VEGF in senescent RPE cells was demonstrated through activated STING signalling under oxidative stress, moreover, senescent RPE cells produced elevated levels of SASP,43, 44 therefore, accelerating pathological neovascularization. The treatment of wAMD targeting oxidative stress and cellular senescence currently includes selective clearance of senescent cells (inhibitors of Bcl-2 or 7-ketocholesterol), inhibition of senescence, or reduction of SASP to suppress chronic inflammation24, 43, 45, 46 .
5 CONCLUSION
In summary, retinal vascular endothelial cell senescence is intricately involved in and promotes the development of pathological neovascularization. Targeting senescence-related signalling pathways and developing drugs aimed at senescence-associated proteins can potentially delay cell senescence. Emerging therapeutic approaches include senolytics, drugs that eliminate senescent cells, and senomorphics that modulate inflammatory secretory phenotypes, which effectively reduce pathological angiogenesis by inhibiting senescence. Therefore, therapeutic strategies targeting endothelial cell senescence may offer promising avenues for slowing or reversing the progression of retinal neovascularization.
AUTHOR CONTRIBUTIONS
The manuscript was drafted by Zehui Shi and Bo Liu. Study supervision was performed by Jinhui Dai and Xiuping Chen.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
PATIENT CONSENT STATEMENT
Not applicable.




