Heterogeneous nuclear ribonucleoprotein A2/B1, a key regulator of myocardial fibrosis
Heart failure is the final symptom of most cardiovascular diseases with a poor prognosis and remains a major cause of death in the United States for 100 years. Myocardial fibrosis (MF), which occurs during the evolution of heart failure, is associated with almost all forms of heart disease. MF is specified by the excessive accumulation or deposition of extracellular matrix (ECM), such as fibrillar collagen, in the myocardial tissue cells, consequently resulting in increased matrix stiffness and abnormalities in normal heart function. Cardiac fibroblast is the primary cell type responsible for the deposition of ECM in the heart, regulating the proliferation of cardiomyocytes, and maintaining the integrity of the matrix network.1-3 Certain pathological conditions cause fibroblast activation and collagen secretion, eventually leading to cardiac fibrosis.3, 4 However, the underlying molecular and cellular mechanisms of cardiac remodelling and dysfunction in heart failure still require further research.
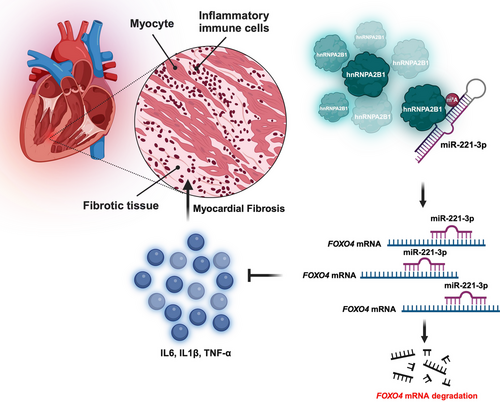
The heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNPA2B1), a member of the hnRNP family, is a nuclear m6A reader recognizing m6A modification in a subset of primary microRNAs (pri-miRNAs).5 The hnRNPA2B1 interacts with DiGeorge syndrome critical region 8 (DGCR8), an essential component of the pri-miRNA processing complex, and mediates the processing of pri-miRNAs containing m6A modification.5 In the acute myocardial infarction and MF, m6A regulators including hnRNPA2B1 are highly upregulated, and the dysregulated m6A signalling is significantly associated with the infiltration of immune cells, suggesting the role of m6A in the development of cardiovascular disease.6 The recent investigation led by Li et al. demonstrated that hnRNPA2B1, a key regulator of MF, regulates the miR-221-3p/Foxo4-mediated inflammatory response and the proliferation of cardiac fibroblasts.7 This study group found that hnRNPA2B1 is also upregulated in MF in an isoproterenol (ISO)-induced model and ISO-treated primary cardiac fibroblasts. The deletion of hnRNPA2B1 significantly diminished the ISO-induced inflammatory infiltration and collagen deposition as well as the cardiac fibroblast proliferation and activation. Furthermore, they found hnRNPA2B1-regulated miRNAs (miR-210, miR-99b and miR-221) in MF. More importantly, they demonstrated that Foxo4, one of the widely expressed forkhead box (Fox) transcription factor O family members, is a target of miR-221-3p of hnRNPA2B1 and hnRNPA2B1/miR-221-3p/Foxo4 axis regulates inflammatory response and myofibroblast activation in the development of MF.
In conclusion, elucidating the role of hnRNPA2B1 as a key regulator of cardiac fibrosis is crucial to demonstrate a new molecular mechanism regulating inflammatory response and myofibroblast activation via miR-221-3p/Foxo4 axis (See Figure 1). The association between m6A modification and hnRNPA2B1-regulated miRNAs has not been studied in this work. Nevertheless, this study identified a key factor responsible for the development of MF and provided an effective therapeutic strategy targeting the new miR-211-3p/Foxo4 axis.
AUTHOR CONTRIBUTIONS
Ji-Hoon Jeong, Kayode Abidemi John, Juyeong Hong: Manuscript writing; Ji Hoon Lee: Final reviewing and editing.
ACKNOWLEDGEMENTS
The authors would like to thank members of the Lee lab for their careful review and helpful suggestions on this work. Ji Hoon Lee is supported by the National Cancer Institute (NCI) R01CA279696.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.




