Intervertebral disc degeneration and regenerative medicine
Abstract
Intervertebral disc (IVD) degeneration is a common phenomenon that affects patients with increasing prevalence with increasing age. Both conservative treatments, such as the use of pain medication or physical therapy, and surgical treatments, such as fusion or disc replacement therapies, are offered to patients. Both non-invasive and invasive treatments have been shown to improve pain and quality of life for patients. This review explores the role of regenerative medicine techniques as a promising therapeutic intervention that can be used before or in combination with conservative therapy and surgery to enhance the treatment process in patients with IVD degeneration or disc pathology. Currently, there are four major modules of regenerative medicine: genetic therapy, platelet-rich plasma therapy, stem cell transplantation and tissue engineering. Several research studies have shown promising outcomes of stem cell transplantation and tissue engineering when combined with either surgical or conservative treatment, resulting in improved pain outcomes. The additional benefit of regenerative medicine techniques, specifically stem cell transplantation, is the potential for treating the root pathology of degeneration. Regenerative medicine techniques also have the potential to either halt or reverse degeneration as opposed to current standards of care for managing symptoms. There is a plethora of current research highlighting the benefits of regenerative medicine techniques; however, there remains clinical concerns and ethical concerns regarding the use of regenerative therapy techniques such as stem cell transplantation in the context of IVD degeneration.
1 INTRODUCTION TO INTERVERTEBRAL DISC PATHOLOGY AND TREATMENT
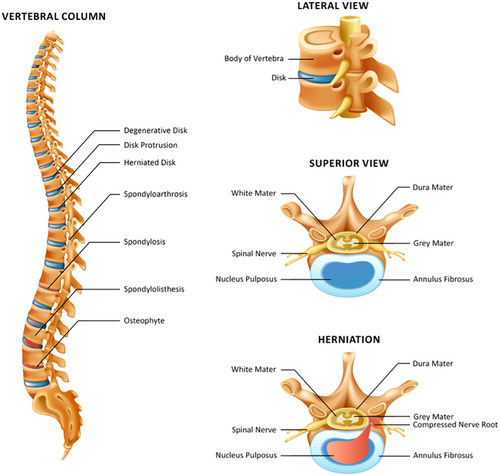
Intervertebral discs (IVDs) serve many functions in the context of everyday movement and support. The IVD is known to be the body's largest avascular structure and is made up of a central nucleus pulposus (NP) surrounded by the annulus fibrosus.1 The NP predominantly consists of type II collagen and proteoglycans, whereas the annulus fibrosus is made up of connective tissue that is organised in adjacent concentrically organised lamella. The NP serves to balance hyaluronic pressure, and the annulus fibrosus balances forces on the spine such as flexion, extension and torsion.1 The structure of the IVD provides support and enhances mobility, but the tissue composition also makes the disc more prone to tissue pathologies such as protrusion, herniations and compressions.1, 2 Given that IVDs are avascular structures, this poses difficulty when it comes to the need for regenerative healing post-injury to restore original tissue structure and composition.1, 2
Disc pathology can range anywhere from degeneration to fracture or traumatic injury. Degeneration resulting from injury or normal wear and tear is a common phenomenon. The process of degeneration is dependent on risk from lifestyles, genetics, stressors from everyday living activities and the normal ageing process.3 Different mechanical, genetic or traumatic stressors on the IVD can result in altered composition of cells and altered connective tissue organisation, which affects normal functioning of the spine, generating pain and limiting quality of life.3 It is estimated that about 45% of individuals younger than the age of 30 years exhibit some form of lumbar IVD degeneration, and that percentage increased to over 80%−90% for individuals between the ages of 50 and 55 years.4, 5 Disc injury or degeneration can lead to increasing spine pathology such as spinal stenosis, radicular pain, back pain or damage to the surrounding structures.2, 4 IVD degeneration or pathology also has a positive correlation with the incidence and prevalence of back pain, which is an increasing public health problem that is currently the fifth most common complaint warranting doctor visits.3, 5
IVD degeneration over time can lead to the development of bone spurs, nerve damage, herniated discs, arthritis, facet arthrosis and spinal stenosis, which are some of the most common presentations of traumatic and non-traumatic spinal pathology. For example, in a study examining cadaveric specimens to extrapolate the prevalence of facet arthrosis specifically by observing the lumbar facet joints of 647 donor bodies, 57% of 20‒29-year olds showed evidence of facet arthrosis, with the percentage increasing to 82% in 30‒39-year olds, 97% in 50‒59-year olds and up to 100% in individuals older than 60 years.6 IVD degeneration and the associated burden of illness are increasing in prevalence and incidence, which begs the need for accessible and effective methods of treatment.7 Current conservative treatments for disc degeneration and pathology include medications and physical therapy exercises along with alternative therapies such as therapeutic massage or acupuncture. Although conservative treatment works for some patients, some patients require more invasive approaches when appropriate pain relief is not achieved or if the patient experiences significantly limited mobility affecting quality of life.8
Patients are offered more invasive methods of treatment when degeneration or injury causes significant effects on quality of life and activities of daily living. There are multiple surgical interventions, which can be divided into stabilisation surgeries, which maintain mobility and stabilise the spine, and decompressive surgeries, which alleviate pressure of degeneration and neurologic symptoms. Stabilisation surgeries include spinal fusions, total disc replacement or combined discectomy and fusion and are routinely offered to patients and entail either fusing vertebra or replacing damaged vertebra.9 Decompressive surgeries include discectomies, facetectomies, foraminotomies or laminectomies, which all entail the removal of a part of a protruding tissue to alleviate symptoms of protrusion, herniation or neurological symptoms (Table 1).
| Decompression surgeries | Stabilisation surgeries |
|---|---|
| Discectomy/microdiscectomy | Spinal fusion |
| Facetectomy or foraminotomy | Artificial disc replacement |
| Laminectomy and laminotomy | Anterior cervical discectomy and fusion |
2 CURRENT BENEFITS AND LIMITATIONS OF THE DIFFERENT SURGICAL APPROACHES FOR IVD DEGENERATION AND ARTHRITIS
Spinal fusions have been one of the oldest performed surgeries for IVD degeneration and IVD pathology or post-traumatic injury. There are different types of fusion surgeries, including posterior lumbar, transforaminal or minimally invasive, which all serve to fuse multiple vertebrae together in order to immobilise damaged disc to minimise pain and provide stability.10, 11 Indications for a fusion surgery, specifically for cervical and lumbar regions, include exhausting conservative management, having radicular pain limiting mobility, displaying deformity on imaging or post-discectomy if osteophytic or spondylosis is present.12 In two systematic reviews looking at the outcome of fusion surgeries for back pain related to disc IVD degeneration treatment, it is evident that fusion surgery resulted in decreased pain and disability in the different patient cohorts from the studies.13, 14 Although there was reported pain reductions in most studies, the limitations of fusion surgeries persisted with patients experiencing limited range of motion due to disc fusion and some patients experienced post-surgical complications such as increased risk of infection or bleeding.13, 14
Total intervertebral disc replacement (TIDR) is another newer approach that can be used in the treatment of IVD degeneration and serves as an alternative to other surgeries, such as fusion surgeries.15, 16 There are different types of disc replacement surgeries spanning from a total replacement to the replacement or augmentation of the nucleus only.17 The indications for a lumbar TIDR included patients who had painful discs for longer than 6 months and who were not responsive to non-surgical treatments.18 TIDR has been shown to be comparable, if not superior, to fusion surgeries when it comes to maintaining patient's full range of movement.19 In a prospective analysis study looking at 5- and 10-year outcomes of TIDR using indices such as the visual analogue scale (VAS) and the Oswestry disability index (ODI), it was reported that there was improvement in both the VAS and ODI scores with 63.6% of patients reporting a satisfactory long-term outcome.20 Although TIDR has potential to benefit patients and maintain mobility, the current limitation of the TIDR is that there are many exclusion criteria, such as patients who have spinal deformities, spondylosis or sclerosis.21
Decompressive and spinal discectomies are two other common surgeries performed for IVD pathology. Decompressions and discectomies can be less invasive surgeries and consequently may have a lower chance of complications than fusion surgeries.22 The benefits of these procedures are that they take less time for recovery, can be less invasive in the management of IVD degenerative complications and show improved pain outcomes in patients.22, 23 In a review of the treatment of spinal stenosis specifically, it has been shown that minimally invasive decompression surgeries improve pain outcomes and mobility with patients recovering faster than patients who have had more invasive procedures.24 Although decompression surgeries and discectomies have fewer complications, there are still reported complications such as bleeding, infection, dural tears and nerve damage that were reported in the 12 studies reviewed.24 Endoscopic discectomies have been shown to be effective in pain management and in a meta-analysis comparing discectomy procedure rates of complication, discectomies have been shown to have lower levels of complications.25, 26 Decompression procedures and spinal discectomies have been shown to be effective procedures, and although they could be performed in a non-invasive manner, they are still limited due to risk of complications and concern for the long-term pain control and mobility.
There is inconclusive evidence regarding which surgical method is the standard of care, as outcomes are often multifactorial depending on the reason for the surgery and patient characteristics.27 Invasive interventions have been shown to reduce the burden of degeneration, and surgery has been shown to increase spinal pain relief, but the duration of results varied. For example, in studies looking at surgical interventions for conditions such as lumbar disc herniation, it has been shown that lumbar disc herniation surgeries resulted in a greater reduction in pain on reported pain surveys than what non-surgical patients reported with the same conditions using conservative therapies, such as pain medication. However, the increased reduction in pain in contrast to the non-surgical patients decreased over time.28, 29 The reported pain reduction in response to surgery equaled that of conservative treatment in the long term, 2 years after surgery. Also, it was noted that if post-surgical pain relief persisted, some patients experienced increased risks of complications such as limited mobility due to the procedure performed. Thus, looking at pain reduction in the context of long-term efficacy or risk complication highlights the benefits and limitations of intervertebral surgical interventions, which shows the need for the incorporation of additional treatments that can increase long-term benefits of surgical intervention.28, 29
Although several conservative and surgical approaches are available in treating IVD pathology, the current challenges include the risk of post-operative complications, limited mobility after surgery and concern regarding long-term benefit. Total disc replacements, when indicated for disc pathology, have been shown to be more effective for patients.30-32 Regenerative medicine integration is a promising treatment intervention alongside surgical intervention and has the potential to enhance long-term recovery and healing. Although regenerative medicine is a feasible treatment, additional research is needed to assess concerns stemming from safety, a lack of clinical trials and the need for more engineering assessments.33 Nonetheless, assessing regenerative medicine techniques that make use of technology such as stem cell integration in the treatment of IVD degenerative diseases has promising potential for improving the efficacy of IVD degeneration treatment.
3 OVERVIEW OF THE DEVELOPMENT OF REGENERATIVE MEDICINE AND STEM CELL THERAPIES
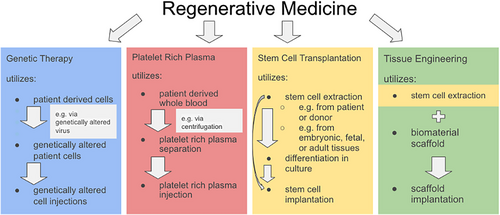
Regenerative medicine regards the method of substituting, fixing or enhancing the regeneration of injured tissue,34 through the utilisation of biological agents with technological methods,35 including biomaterials, scaffolding, tissue engineering, gene therapy and stem cell transplantation.36 Metal nanomaterials can also be utilised as regenerative techniques to promote angiogenesis, the formation of new blood vessels to support the survival and growth of tissue by transporting oxygen and nutrients.37 Tissue engineering refers to the application of engineering towards developing biological substitutes to restore, maintain or improve tissue function,38 which utilises scaffolds, stem cells and biomaterials that act as a template for providing the supply of factors required for proliferation and differentiation of cells.39 Genetic therapy regards the ability to correct mutated genes for therapeutic treatment.40 Platelet-rich plasma (PRP) is the autologous liquid fraction of peripheral blood that contains platelet concentrations over the baseline and can be used in regenerative medicine since platelet growth factors support the phases of wound healing including proliferation and remodelling.41
Stem cells are one of the basic categories of cell types in the human body42 and are characterised by the ability to develop from a single cell and to divide indefinitely, with the potential to become mature differentiated cell types. Stem cells are specialised organs that assist in tissue repair and are classified based on their origin—embryonic stem cells (ESCs), adult stem cells and foetal stem cells, mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs)—and their capacity for differentiation—listed by decreasing differentiating potential: omnipotent, pluripotent, multipotent, oligopotent and unipotent.43 The stem cells used in therapy can be autologous (cells derived from the patient) or allogeneic (cells derived from another person).44 Stem cell therapy has become a very promising and developed topic within research.45 In the 1960s, the first stem cells were recognised by Drs. James A. Till and Ernest A. McCulloch, when stem cells originating from mouse bone marrow cells, displayed pluripotency—the ability to give rise to all cell types in an adult46 by differentiating into a variety of cell types.47 In the late 1990s, the isolation of ESCs was later prepared from human lineage, introducing a new era of stem cell research and its application to regenerative medicine for human life.48
Different cell sources can be used for IVD degeneration, including IVD-derived cells (NP-derived cells), chondrocyte-like cells, MSCs, iPSCs and ESCs, which have been analysed for cell transplantation in disc regeneration therapy.48, 49 Although initial results show the positive effects of cell injection strategies for IVD regeneration, the avascular niche of the IVD limits survival and adaptation for transplanted cells (2015).49, 50
4 EFFICACY AND SAFETY CONSIDERATIONS OF REGENERATIVE MEDICINE
Stem cells, specifically ESCs and iPSCs, have demonstrated promising potential applications towards regenerative medicine.51 Studies have found that MSCs are safe and have potential effectiveness for alleviating pain and reducing cartilage regeneration in the management of osteoarthritis. Stem cell therapy has been found to be superior to traditional treatments for knee osteoarthritis (KOA) without inducing side effects.52 However, some systematic reviews suggest that there is low quality of evidence for the potential of MSC therapy on clinical outcomes. Also, there has been no evidence supporting the most effective source of MSCs for osteoarthritis management. Therefore, MSC therapy is recognised as a safe and feasible option for osteoarthritis management, yet with many questions remaining, it is not recommended as a first-line treatment.53 Another concern for MSC therapy success and efficacy arises in the circumstances of harsh microenvironments created by conditions, such as degenerated IVDs. To overcome such obstacles, it is recommended to develop techniques, including genomic techniques, to precondition and prepare MSCs for survival in such harsh circumstances.54
Tissue engineering and scaffolding have demonstrated limited clinical success, thus requiring further improvement, specifically regarding the biocompatibility of natural and synthetic materials in scaffold production, for effective use.39 An ideal scaffold for IVD replacement should have good biocompatibility with the shape, structure and mechanical properties similar to those of the IVD to restore its anatomy and function.55 Also considering that this method demonstrates higher costs and less familiarity to its users, further advanced research is suggested for its application in regenerative medicine.56
Genetic therapy has been recognised for improved safety and efficacy as genetic manipulation has become more precise with improved specificity from new generations of targeted nucleases. However, considering the relative novelty of gene therapy and gene editing, the highest possible standards of safety must be diligently maintained by assessing each step of the editing process due to many potential variables that may interfere with safety and specificity.57
PRP is another potential regenerative medicine technique; however, concerns have been shared regarding PRP therapy because of the diversity of preparation methods and resulting products, along with contradictory results found among the few high-quality randomised controlled trials conducted.58 An article expressed caution for regenerative medicine by acknowledging the emerging technologies and their disruptive nature, complicating the translation of regenerative therapies into clinical applications.59 Moreover, it has been mentioned that regenerative therapy studies have no consistency among trials, insufficient documentation, an absence of standardisation for cellular materials and inadequate methods of structuring research, leading to selective reporting and the disregarding of complications.60 This complicates comparison among studies and hinders further progression of studies, especially since not all published clinical trials adhere to the International Society for Cellular Therapy or International Society for Extracellular Vesicles guidelines.61 Thus, it is recommended to improve the translation of regenerative medicine therapies by further identifying the mechanisms of action from each therapy and instilling more rigorous and long-term studies to conclude the safety and efficacy of regenerative therapies.59, 60 Ultimately, regenerative medicine displays significant potential, yet requires further research to safely translate therapy into the clinical setting, especially in the context of pathologies such as IVD degeneration.62
5 CLINICAL STUDIES OF REGENERATIVE MEDICINE WITH IVD PATHOLOGY
Currently, there is no treatment available for the irreversible degeneration of lumbar IVDs. However, the utilisation of MSCs in regenerative medicine treatment has been researched in clinical studies. For example, NP has been recognised for its importance in preserving disc structure, by slowing down further disc degeneration, with its viability being significantly upregulated by its direct contact with MSCs. A 3-year prospective clinical study was conducted on nine patients aged between 20 and 29 years, with a level of Pfirrmann's grade III disc degeneration. The viable NP cells from the fused disc were cocultured with autologous bone marrow-derived MSCs. One million of the activated NP cells were then transplanted into the degenerated disc adjacent to the fused level, a week after the first fusion surgery. As a result, none of the cases reported any low back pain and one case demonstrated mild improvement to the transplanted discs on magnetic resonance imaging (MRI), while the other cases displayed no negative effects to the transplanted discs. Furthermore, the study provided promising findings suggesting the minimal efficacy of this treatment to slow down further regeneration of IVDs.63
Another case study performed therapeutic IVD regeneration therapy via cultured MSCs from marrow fluid on two lumbago patients who displayed lumbar spinal canal stenosis and instability with the vacuum phenomenon. The stenosed spinal canal was fenestrated during surgery, and pieces of a collagen sponge containing the autologous MSCs were grafted to the degenerated vertebral discs. Two years after the surgery, both patients demonstrated improvements in the vacuum phenomenon on radiograph and computed tomography. Moreover, lumbar disc instability improved, made clear on the roentgenography and both patients experienced alleviated symptoms. Therefore, using MSCs for disc regeneration provided suitable results as a minimally invasive therapy.64
Furthermore, 10 patients dealing with low back pain from lumbar disc degeneration and intact annulus fibrosus were analysed after the treatment with autologous expanded bone marrow MSC injected into the NP area. Although MRI did not demonstrate recovery of disc heights, water content increased after 12 months of the treatment, and patients relayed feeling 85% of their maximum after 3 months of treatment. In other words, MSC therapy was determined to be a simpler and more conservative intervention for lumbar disc degeneration, without surgery and with effective pain relief.65
A 10-year follow-up of a phase I/II clinical trial on 11 patients undergoing spinal fusion with autologous MSCs embedded in tricalcium phosphate confirmed the safety of the procedure with no evidence of tumour, infection or inflammatory reaction possibly related to the MSC therapy. Additionally, the radiological analysis indicated that all cases of spinal fusion improved over time, as low back and radicular pain, according to the ODI, remained lower than before the intervention. Ultimately, the study concluded that utilising tricalcium phosphate-embedded autologous MSCs with lumbar posterolateral arthrodesis is safe and could provide long-term benefits for 10 years.66
Different from researching MSCs as regenerative therapy for IVD, one case study observed the potential of PRP injections into affected facet joints and surrounding ligaments involving the lumbar, thoracic and cervical spine. The five patients received a series of three PRP injections, and follow-up examinations were reported after 6−12 months. After the second injection, the percentages of patients who experienced symptom improvement were 60% for patient 1, 60% for patient 2 and 40% for patient 3. Patient 4 recognised 70% symptom improvement and functional status following the third injection, and patient 5 recognised 66%−70% symptom improvement and functional status at 6-month follow-up. Ultimately, it was concluded that PRP injections could potentially be a viable treatment option for spinal pain related to facet joints. However, further research with more patients and longer follow-up periods is highly recommended.67
6 REGENERATIVE MEDICINE AND TISSUE INTEGRATION
Tissue integration with existing bone structures can be an overlooked aspect of regenerative medicine but is important for restoring the functionality of an injury. For instance, regeneration of missing portions of the mandible that resources the shape of the jaw but does not reconnect with the stump tissue may hinder the ability of the mandible in mastication and speech.68
Data suggest that nanotopographic surfaces—surfaces with specific features present at the nanoscale69—display the potential to benefit from the utilisation of stem cells for skeletal regeneration. However, there is a poor understanding of the mechanisms by which nanotopographies affect cell behaviour and of the most relevant bioactive molecules that functionalise nanotopographic surfaces for optimum osteogenic potential.70
The integration of biomaterials with the surrounding original tissue is vital for the effective functionality and long-term performance of the tissue. Surgical integration of tissues utilises many methods, including sutures, cyanoacrylates, bioglue and fibrin glue, which hold some efficacy but lack in biocompatibility and bonding strength. However, integration between biomaterials and tissues has succeeded through a method utilising chondroitin sulphate, a polysaccharide found in cartilage, allowing for anti-inflammatory activity, water and nutrient absorption and improved biological activity for the restoration of arthritic joint function.71
7 REGENERATIVE MEDICINE IN IVD TREATMENT
It has been shown that regenerative medicine stands as a focus of research across diverse medical domains, with particular emphasis on its application in IVD treatment to address leading causes of disability such as back pain.72, 73 Presently, prevailing therapeutic modalities for IVD injuries predominantly entail invasive approaches, a practice that, despite its widespread use, may not yield optimal long-term efficacy, and is associated with higher risk of complications.74, 75 Numerous studies have delineated the potential efficacy of intradiscal injection of stem cells as a therapeutic intervention for human degenerative disc disease, both in vitro using nude mice and in vivo with no safety concerns.76, 77 The endorsement of utilising stem cells, as indicated by preliminary data from several studies, has propelled the medical community to contemplate their application in regenerative medicine as an alternative to invasive procedures.78, 79
Current use of stem cells for IVD regeneration include iPSCs, muscle-derived stem cells and haematopoietic stem cells.80 The current methods of using stem cells for IVD regeneration include the direct injection of undifferentiated or pre-differentiated cells into the IVD, known as cell therapy. Another strategy involves creating constructs by combining stem cells with a viscoelastic hydrogel, constituting a tissue engineering approach. Additionally, a technique known as ex vivo gene therapy is employed, wherein target genes are transfected into stem cells, followed by the injection of these modified cells into the IVD.81-84
8 STEM CELL INTEGRATION
IVD degeneration impacts both the quantity and quality of intervertebral disc stem cells (IVDSCs). Ageing and degeneration lead to a significant decrease in stem/progenitor cell markers within disc tissues, indicating IVDSC depletion.85 Studies have also shown a decline in specific progenitor cell frequencies such as Tie2+ and GD2 cells with age and disc degeneration, suggesting progenitor cell exhaustion.86 However, precursor cells (Tie2+ GD2−) have shown the ability to transition into progenitor cells (Tie2+ GD2+) under simple culture conditions. In addition to these cellular dynamics, scholarly literature has established that chondrocytes located within the endplates are notably susceptible to degeneration as the IVD experiences mechanical wear and tear.87, 88
Among the various effective approaches in regenerative medicine, the utilisation of MSCs shows significant scientific promise for addressing IVD degeneration and stem cell depletion.89 MSCs play a role in rejuvenating degenerating disc tissue through diverse mechanisms: they replace lost or impaired cells by directly differentiating into disc-specific cells, aiding in the formation of the extracellular matrix (ECM); they promote tissue regeneration indirectly by releasing growth factors; and they immunologically regulate the inflammatory response.90 Thus, at a molecular level, MSCs possess the capability to transform into NP-like cells, demonstrating the capacity to multiply and replenish the deteriorated IVD directly. They can also activate the existing NP cells or administer desired gene products.91-93 In addition, several studies have emphasised the advantages of utilising stem cells for the regeneration of IVDs in animals. Notably, at the molecular level, a meta-analysis revealed increased expression of type 2 collagen, enhanced disc height and heightened MRI T2 signal in these studies, all of which are positive indicators of tissue regeneration.94-96
Furthermore, alongside the utilisation of stem cells in regenerative medicine for IVD degeneration, the scientific community is investigating PRP as a potential therapy for alleviating pain and symptoms associated with osteoarthritis and other skeletal disorders. Within the realm of research, one study revealed promising outcomes in the management of mild to moderate KOA through the application of PRP. This study indicated that 68% of patients experienced more than 50% improvement in pain, stiffness and knee joint function.97 Additionally, Filardo et al.’s research suggested notable clinical enhancement with PRP injections observed during a 1-year follow-up period.98
While acknowledging the promising potential of PRP therapy in IVD regeneration, there is currently a lack of studies or clinical treatments directly related to its application. Therefore, this paper will primarily concentrate on the utilisation of stem cells for addressing IVD degeneration. Regenerative medicine therapies, particularly stem cell treatment, have shown efficacy in alleviating pain and symptoms, counteracting the degenerative process, slowing down age-related changes, preserving spinal structure and maintaining mechanical functionality.99, 100
9 REGENERATIVE MEDICINE FOR ARTHRITIC CHANGES
Chronic back pain resulting from facet joint syndrome is a prevalent and incapacitating issue. Recent research studies suggested that the use of stem cell therapy could potentially aid in relieving pain associated with facet arthritis and promote healing. Specifically, it has been indicated that regenerative cells derived from the patient's own unmodified adipose tissue-derived regenerative cells (ADRCs) offer numerous advantages.101 These cells have the ability to transform into different tissues and demonstrate significant anti-inflammatory properties. The referenced study involved 37 patients who received ADRC injections and underwent follow-up assessments at 1 week, 1 year and 5 years post-treatment. The results of this study demonstrated that patients with facet joint syndrome who were treated with unmodified ADRCs experienced long-term improvements in their quality of life.101 Additionally, a very recent case study involved a patient with a 13-year history of chronic lower back pain who was resistant to conservative treatment. The decision was made to treat the patient with umbilical cord-derived MSCs, and 87 million MSCs were infused intravenously. This study demonstrates, for the first time, that injecting MSCs into the lumbar facet joints and epidural space significantly improves lower back pain and can alleviate symptoms in other spinal regions without incurring the risks associated with intradiscal injections or the epidural use of corticosteroids.102
Furthermore, in addition to the therapeutic effects previously discussed regarding stem cell therapy for IVD degeneration, stem cells possess the capability to directly modify the mechanical characteristics of NP cells. This modification has the potential to reduce cellular and matrix stiffness, consequently enhancing the viability of NP cells.103 Overall, studies have demonstrated that coculturing MSCs with degenerated NP cells resulted in a notable decrease in the mechanical moduli of the NP cells such as stiffness. Simultaneously, this coculture approach led to increased cell proliferation, as well as elevated expression levels of collagen type II and aggrecan.104
Other notable advantages linked to utilise stem cell therapy in facet arthritis include alleviating the escalating socioeconomic burdens associated with this condition and effectively managing the pain associated with facet arthritis.105 Although the advantages of regenerative medicine in addressing facet arthritis are apparent, several limitations must be considered. Notably, the research specific to this topic is currently limited. Moreover, facet arthritis represents a broad term, and addressing its various manifestations requires a reduction in heterogeneity or an expansion of research efforts within the field to therapeutic methods tailored to specific cases. Other limitations include ethical concerns surrounding the use of stem cells, especially ESCs, for therapy. This raises questions about the concept of human personhood and how it is defined during the embryonic stage.106
Furthermore, despite some initial data suggesting the safety of stem cell therapy, there remains a question of interest regarding whether the use of stem cells for therapy could result in undesirable cell differentiation, potentially leading to malignancies. This concern arises from the potential role of MSCs in bridging the connection between the immune response against tumours and the formation of new blood vessels in malignant diseases, consequently supporting the progression of tumour growth and metastasis.107
10 REGENERATIVE MEDICINE FOR BONE GROWTH
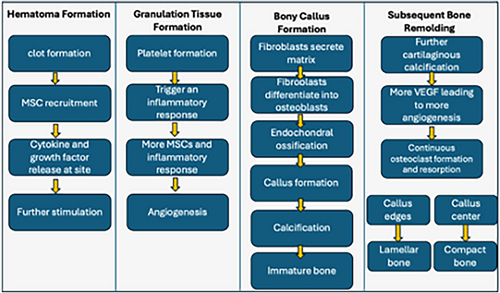
Although stem cell therapy has been deliberated concerning IVD degeneration throughout this paper, it is imperative to assess its therapeutic potential in the context of bone growth therapy. Bone growth typically entails a more extensive vascular network compared to IVDs, which are avascular structures. Among the many factors involved in bone growth, osteoblasts are pivotal cells responsible for the synthesis and formation of bone tissue. They produce various ECM proteins, including osteopontin, type I collagen, alkaline phosphatase and osteocalcin.108 The structural unit of bone is the osteon, which forms as osteoblasts interact during the bone formation process. However, bone healing or regeneration involves distinct stages. These stages include primary healing, characterised by the precise reduction, alignment and fixation of bony fragments under compression, minimising motion at the fracture site.108 Alternatively, secondary healing stages, summarised in Figure 1, involve the sequence of haematoma formation, granulation tissue formation, bony callus formation and subsequent bone remodelling.109
The regenerative capacity of bones relies significantly on MSCs made in the bone marrow, leading to the differentiation of osteogenic cells. Additionally, the introduction of specific external elements, such as dexamethasone, β-glycerol phosphate and ascorbate, has been observed to influence the differentiation process.110 Other factors that induce the differentiation of MSC include transforming growth factor beta (TGF-βs), insulin-like growth factor-1, fibroblast growth factor-2, bone morphogenetic proteins and other growth factors.111, 112 In addition, genetic manipulation, such as the overexpression of adenovirus-mediated SRY- box transcription factor number 9 (SOX9) (crucial transcription factor for guiding the specialisation of chondrocytic lineage), has shown to enhance MSC differentiation in vitro and in vivo (Figure 2).113, 114
Some of the initial applications of MSC therapies demonstrated benefits in terms of pain relief. One year after transplantation, nine out of 10 patients experienced pain alleviation, along with an improvement in disc hydration, as indicated by T2-weighted sagittal images.64 While MSCs hold significant promise in the field of regenerative medicine, concerns are arising regarding the need for quality control and standardisation of cells intended for clinical use.115 Other limitations with the use of MSC include their scarcity in the bone marrow of adults (only accounting for less than 0.01% of bone marrow cells) and although this issue is thought to be mitigated through culturing the MSC, the chances of irregularities in both genetic and epigenetic factors are still of concern as mentioned previously.90 Moreover, acquiring MSCs from a donor rather than utilising them those from patients under many conditions introduces considerable variability in proliferative capacity and lifespan. This variability poses challenges in standardising the care protocols associated with MSC therapy.116
Another constraint associated with MSC therapy is the absence of complete purity in the utilised MSC sample. Typically, traces of various committed progenitor stem cells are still present, further contributing to the observed heterogeneity in MSC treatment.117 Ultimately, another constraint lies in the fact that while MSC injections, combined with glucocorticoids, methylene blue and cytokine inhibitors, can provide short-term relief by reducing inflammation and alleviating symptoms, sustaining their effectiveness over an extended period is challenging due to their limited half-lives.118 The utilisation of stem cell therapy, particularly in the context of MSCs, along with factors that induce differentiation, holds significant promise for facilitating bone repair. Although further research is required to elucidate the molecular mechanisms and specific stages associated with bone regrowth, there is substantial potential for the application of this therapy in the field (Figure 3).
11 COMPARATIVE ANALYSIS OF SURGICAL AND REGENERATIVE APPROACHES
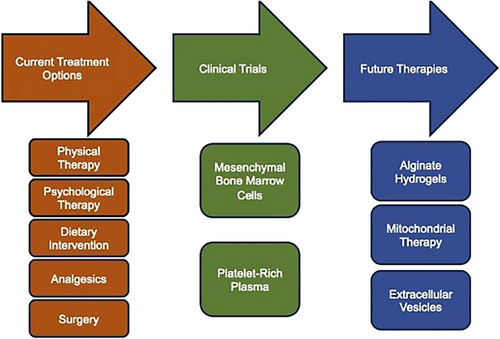
There is a need for IVD degeneration treatments that target bone repair and continued regeneration of tissue. Radiological evidence has shown that IVD degeneration is correlated with lower neck and back pain.119 Several conservative therapies can be employed for pain derived from IVD degeneration such as physical therapy, psychological therapy, dietary intervention or analgesics that target symptoms not pathology mechanisms.120, 121 This is of important note since lower back pain is a leading cause for the prescription of opioid analgesics in the United States.121 However, conservative treatment, such as the use of opiods, are palliative, targeting symptoms of IVD degeneration instead of its underlying aetiology. Similarly, surgical strategies for IVD degeneration, such as fusion and total joint replacement, are commonly employed to replace damaged tissue and provide stability.122, 123 However, the efficacy of surgical strategies for reducing long-term pain and disability in IVD degeneration is questionable and can even accelerate degeneration of adjacent levels in the long term.89, 124
Compared to surgical and only conservative outcomes, regenerative techniques are more attractive because they are less invasive and can delay or even reverse IVD degeneration. The cause of IVD degeneration is multifactorial and includes both genetic predisposition and behavioural and environmental factors.125, 126 Techniques such as intradiscal injection of MSC therapies and PRP seek to target IVD degeneration on a cellular level.127-129 Degenerating IVDs are characterised by an increase in senescent cells.130 Regenerative procedures aim to directly replace these cells or recruit new progenitor cells to the IVD via chemical signalling.131, 132
The potential application for cell therapies and PRP is much wider than surgery. Undoubtedly, there will be severe or traumatic cases where surgery is required for structural repair. However, cases of mild and moderate IVD degeneration are prime candidates for regenerative techniques.133 These techniques could also be monumental in improving the quality of life of patients who are too frail for surgery.134 Some studies indicate positive outcomes for supplementing IVD surgeries with cell therapy.135 Overall, regenerative and surgical treatments are not mutually exclusive. Treatment of IVD degeneration should be viewed as a spectrum of least to most invasive interventions with conservative therapies being followed by regenerative therapies before finally considering surgery.
12 CHALLENGES OF TRANSITIONING FROM SURGERY TO REGENERATIVE MEDICINE
The primary challenge of transitioning from surgery to regenerative medicine for the treatment of IVD disease is the dearth of clinical data.120 Much like surgical techniques, regenerative techniques are heterogeneous, including biomaterials, gene therapy, cell therapy, growth factors and tissue engineering.136 The efficacy of each of these methods in alleviating pain and reversing IVD degeneration must be rigorously investigated to determine their use cases. Meta-analyses and systematic reviews have elucidated that most clinical trials focused on the use of cell therapies and PRP are inconsistent in their experimental methods and have relatively short follow-up times.120, 137
The paucity of long-term data considerably diminishes efforts to integrate regenerative techniques into common practice. Intradiscal injection of PRP has the most evidence for long-term safety. A randomised controlled trial of intradiscal PRP found that the injections were able to provide pain relief and delay surgery for lumbar IVD degeneration as far as 5−9 years post-injection without significant adverse events.138 This is the only long-term randomised controlled trial of PRP and most other regenerative therapies have no comparable evidence.137
Another challenge in the transition to regenerative medicine in the treatment of IVD is inconsistent measurement of outcome. For pain measurement, some studies use a verbal pain scale,139 others use the VAS,115 while others use the Japanese Orthopedic Association scale.135 Disability, radiological changes and treatment dosing results are also inconsistent.120, 137 To further support the adoption of regenerative techniques, procedures and outcome measurements need to be standardised.
13 EMERGING TECHNOLOGIES IN IVD RESTORATION
Despite the limited number of clinical applications of regenerative medicine techniques for IVD degeneration, the landscape of preclinical research on IVD degeneration is immense. Research into tissue engineering provides new biomaterials, such as alginate hydrogels, to restore or replace IVD tissue.140 In addition to tissue replacement, further investigations into the cellular mechanisms and pathways involved in the function and dysfunction of IVDs and related structures are providing targeted therapies for restoring IVDs, such as mitochondrial141 and exosome therapies.142 These technologies could be valuable tools that increase the repertoire that providers have to treat the root cause of IVD degeneration instead of focusing on palliative care.
Biomaterials are already used in the context of structural restoration.143 However, the primary effort of new tissue engineering work is to restore of the cellular environment in damaged tissue, such as degenerating IVDs.144 Alginate hydrogels are a promising new biomaterial for the treatment of IVD degeneration because of their biocompatibility, low toxicity and anti-inflammatory effects.140 Alginate hydrogels are made from the linear polysaccharides of bacterial cell walls.145 They can mimic the IVD extracellular membrane via freezing‒thawing-induced cross-links in alginate chains.146 In addition to this structural similarity, alginate hydrogels can also be chemically modified to increase their adhesion, allowing them to withstand the forces experienced in the vertebral column.147, 148 These chemical modifications can also be selected to stimulate bioactivity that supports tissue regeneration.149 Alginate hydrogels also have a high capacity to retain water, allowing them to deliver cell or drug therapy to target tissues.150 Alginate hydrogels have the potential to be intradiscally injected or completely replace damaged IVDs.151 This would provide the structural restoration of a joint replacement or fusion as well as the regenerative therapeutic effect of cell therapy delivery. In vivo translational studies of alginate hydrogels have shown promise in safety and efficacy for decreasing inflammation and preventing degeneration of IVDs.152, 153 Overall, tissue engineering with biomaterials such as alginate hydrogels provides structural and regenerative benefits that could revolutionise the treatment of moderate to severe IVD pathology.
Although largely avascular, mitochondrial-mediated metabolic adaptability plays a role in IVD degeneration.141 Mitochondria play a role in signalling and regulation, which is key to the function of IVD cells.154 Biomarkers of IVD degeneration include calcium dysregulation, cytochrome C leakage and increased reactive oxygen species, which are all consequences of mitochondrial dysfunction.155 This makes mitochondrial therapy an attractive supplement for the treatment of IVD degeneration. To combat the consequences of oxidative stress in IVD cells, such as cell senescence and calcification, patients could be treated with antioxidant molecules.156 There is a gap in current knowledge about the effects of antioxidant molecules on IVD degeneration, which, if filled, could lead to treatments to supplement IVD degeneration patients.
Another emerging technology in the regeneration of IVDs is exosome therapy, which employs extracellular vesicles (EVs) derived from cells. Investigations on the mechanism of MSC-based therapies for IVD degeneration have elucidated that in addition to differentiating into NPCs, MSCs also release anabolic biomolecules that support ECM synthesis and promote NPC function.157 Exosome therapy seeks to strip away the superfluous material delivered in whole-cell therapies such as MSC injections by delivering only the biomolecules that matter for tissue regeneration.158 A plethora of research is being conducted about the interactions between EVs and cells throughout the body, characterising their role in regulation and communication.159 Both exosomal surfaces and payloads contain ligands that bind to receptors and modulate signalling pathways.160 EVs are an attractive potential therapeutic because they are evolutionarily designed to deliver a mixture of pertinent biomolecules to targeted tissue and stimulate regeneration.161 Additionally, because EVs contain no recombinant molecules, they have low immunogenic potential. Much like cellular therapies, EVs could be injected intradiscally to provide a less invasive alternative to surgery for patients with moderate IVD degeneration.
Recent research has uncovered how MSC-derived EVs deliver many miRNAs and growth factors that inhibit NPC apoptosis and suppress inflammation.162 MSC exosome-delivered miR-410 alleviates anti-pyroptosis and prevents IVD degeneration by inhibiting the NLRP3 pathway.103 Induced MSCs contain miR-105-5p, which increases anabolic markers of NPCs in vivo and restores IVD height in a rat model.163 Human placental MSCs also contain anti-miRs, such as antagomir-4450, which suppresses miR-4450 in vitro and in vivo and lowers inflammatory factors such as matrix metalloproteinase- 13 (MMP-13), interleukin (IL)-6 and IL-1β.164 By parsing through MSC EV contents and their effects on IVD cells, future therapies that deliver only the therapeutic MSC-derived molecules can be developed.
In addition to MSC-derived EVs, exosomes from other cell types may also play a role in future therapies for IVD degeneration. Adipose-derived stem cells are a source of macrophagic EVs that modulate inflammation.165 The isolation of macrophagic EVs whose signal polarisation and upregulation of M2 macrophages could stimulate anti-inflammatory responses, a key factor in IVD degeneration.166 iPSCs are another source of EVs that could be used to scale the production of exosome therapies for cases where autologous MSCs are not available. Pluripotent stem cells differentiated into MSCs produce EVs that can regenerate rat NP cells in vitro via delivery of miR-105-5p miRNA.167 This creates hope for future therapies cultured from iPSCs delivering biomolecules tailored to the specific cellular environment that contributes to patient's IVD degeneration (Figure 4).
14 CONCLUSION
Overall, the future of regenerative therapies for IVD degeneration is bright. A better understanding of the biomarkers and cellular environment underlying IVD degeneration is key. In the realm of biomaterials and tissue engineering, alginate hydrogels shine as a model for a biocompatible material that provides structural and chemical support to IVDs. Additionally, a better understanding of mitochondrial dysfunction and its contribution to IVD could lead to therapies tailored to combatting oxidative stress that characterises IVD degeneration. In the future, all MSC therapies may be replaced by MSC-derived EVs. More developed connections between different EVs, their contents, and IVD cells will be crucial to expanding the credibility of cellular therapies and the development of exosome therapies that could be safer and more specific.
AUTHOR CONTRIBUTIONS
All the authors have approved the manuscript for submission. The content of the manuscript has not been published or submitted previously.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
There are no issues relating to journal policies.




