Roles of telocytes dominated cell–cell communication in fibroproliferative acute respiratory distress syndrome
Yonghua Zheng and Songshan Cai contributed equally to this article.
Abstract
Telocytes (TCs) are a new type of interstitial cell identified in multiple tissues of mammals, including the human lung, and mediate homocellular or heterocellular cell-cell communication. Acute respiratory distress syndrome (ARDS) is characterized by acute hypoxemia respiratory failure and combined with direct and indirect lung injury, which is induced by pneumonia, sepsis, burns, etc. Pulmonary fibrosis is a progressive lung disease that occurs due to increased fibrosis of lung tissue in response to chronic injury of the epithelium and gets more and more attention as a well-recognized sequela of ARDS or mechanical ventilation. However, the existing intervention measures could not prevent the progression of pulmonary fibrosis. Although the protective effect of TCs in acute lung injury had been demonstrated in both cellular and animal models in previous studies by our or other researchers, the roles of TCs mediated cell-cell communication in fibroproliferative ARDS is unclear. This review is aimed at integrating our understanding of TC-mediated cell–cell communication in lung diseases with pulmonary fibrosis after ARDS.
1 INTRODUCTION
Fibroproliferative ARDS was proposed as the coronavirus disease 2019 (COVID-19) pandemic has caused numerous ARDS patients, and the chronic consequences of this disease are emerging.1 Lungs are the primary source of infection and injury in ARDS.2 First, the exudative phase of ARDS is the initial response to acute lung injury causing disruption of the alveolar epithelial-endothelial barrier in which edematous flooding of the alveolar and interstitial compartments occurs.3 Secondly, the proliferative phase ensues to repair injury through re-establishment of the alveolar barrier with clearance of exudative fluid. While there have been remarkable advances in revealing the complex mechanisms underlying these initial phases of ARDS.4 Thirdly, the fibrotic phase remains not fully understood.5 Moreover, therapeutic methods remain limited, as current treatment strategies can only delay the progression of the disease. While idiopathic pulmonary fibrosis (IPF) and fibroproliferative ARDS are distinct entities, the underlying pathobiology of these diseases almost certainly share similarities.6 Extracellular matrix (ECM) abnormally deposits in multi-focus of the lung tissue, accompanied by epithelial and endothelial cell injury, alveolar structure and lung compliance damage, ultimately leading to respiratory failure and death in the pathological process of pulmonary fibrosis.7
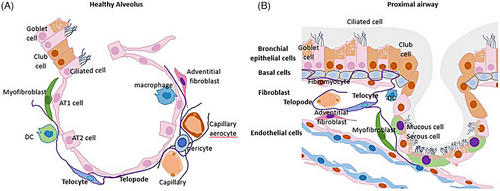
Same as ARDS, therapeutic options for pulmonary fibrosis are also limited as current therapies could only delay disease progression. Recently researchers have tried to treat pulmonary fibrosis with stem cells or extracellular vehicles (EVs).8 The understanding of the mechanisms of modulation in cell-cell communication among multiple cell types and lung tissue microenvironment during the fibrotic phase of ARDS is the basis for new therapeutic options. Telocytes (TCs) are a distinctive interstitial cell type which performs three-dimensional (3D) stromal networks in lung tissue of mammals, including humans, mice rats, etc. (Figure 1A,B), and have been demonstrated could improve lung injury and promote repair in ARDS mice models and improve lung tissue fibrosis after lung transplantation in rat models. However, the function and mechanism of TCs and their derivative substance in fibroproliferative ARDS were still unclear. Reports showed the ability of TCs to mediate intercellular communication with mesenchymal stem cells, epithelial cells and endothelial cells by producing cytokines or exosomes and alleviating inflammation and lung injury induced by LPS or ventilator.9 Owing to their close relationship with stem cells, TCs are also supposed to contribute to tissue repair/regeneration.10 Additionally, TCs are also closely associated with infiltrated immune cells in the stroma.11 TCs have been reported during the fibrotic remodelling of multiple organs in various diseases, including scleroderma, Crohn's disease, ulcerative colitis, and liver fibrosis, as well as in chronic inflammatory lesions like primary Sjögren's syndrome and psoriasis. Recent reports showed that TCs could affect the proliferation and inflammation of fibroblasts in a non-contact coculture system.12 Notably, there is evidence to support that TCs could help in preventing abnormal activation of immune cells and fibroblasts, as well as in attenuating the altered matrix organization during the fibrotic process.
In this review, we summarized the current knowledge about the relationship between TCs and pulmonary fibrosis. We emphasized the roles of TCs in the pathogenesis of fibroproliferative ARDS and their therapeutic potential. Targeting TCs during different stages of fibroproliferative ARDS, might reduce tissue damage, prevent fibrosis, promote regeneration, and thus prevent irreversible lung tissue damage. In addition to exogenous TCs transplantation, enhancing the growth, survival or other specific functions of TCs by targeting gene modification or pharmacological pretreatment to different stages of progression may provide new strategies for the prevention and treatment of ARDS-related fibrosis.
2 STRUCTURE OF TC-MEDIATED CELL COMMUNICATION
TCs' functional regulatory roles have been described in various diseases, such as myocardial infarction, gallstone disease, endometriosis, hydronephrosis and psoriasis, especially in experimental acute lung injury.13 Recent studies considered that TCs were related to immune system modulation.14 TCs orchestrated cell-cell communication was crucial modulators of intercellular signalling through adjacent epithelial, nervous, vascular and immune cells or long-distance communication by direct contacts or releasing cytokines/EVs for modulation at long remoteness (Figures 1 and 2).15-17
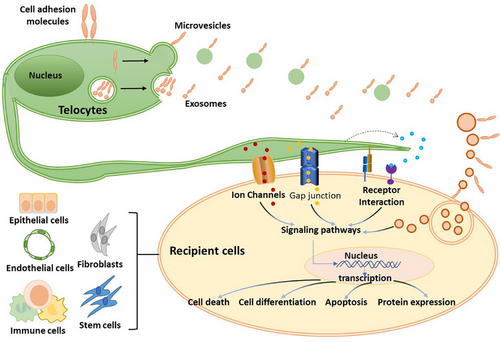
2.1 Cell junction formed within the contact site of TCs and other cells
3D stromal networks of TCs in pulmonary interstitium were formed with fibroblasts, immune cells, neural cells, smooth muscle cells and blood capillaries through telopodes, namely the long processes of TCs.18 Cell-cell contact indicated direct interaction, and TCs are highly related to the scaffold roles in the organ. The cell contacts are mainly located along their long and thin processes and might act as mechanical cell-to-cell attachments or sites of intercellular communication.17 Extensive appositions of the plasma membrane were found between the TCs and immune cells indicating the contact interaction between these two cell types.19 Although cell contact between TCs and neurons was never observed under the transmission electron microscope (TEM), TCs could respond to some neuronal mediators.20 Cell junctions had been observed in previous studies. Pro. Vannucchi described the contact between TCs, macrophages and smooth muscle cells through TEM20 Telopode was considered crucial for the cell junction formation between TCs and other types of cells.21
Telopodes (Tps) were significant in TCs-dominated cell-cell communication22 (Figure 2). A synaptic junction between Tps and the presynaptic axolemma was observed and described in detail as a heterocellular junction between TC and terminal edge cutaneous nerve.21 Cell junction is the basis of intercellular Channels, and the dilated segments of podoms on Tps with enrichment of mitochondria and lack of ER indicated that there might be mitochondria and ER transport within the synaptic junction formed by Tps with other cells.23 Thus, through extended contact, TCs might not only maintain the position of various types of cells in the niche but also create a microenvironment to shelter the cells in tissue from possible adverse conditions.
2.2 Roles of exosomes in TCs mediated cell communication
Extracellular vesicles (EVs) are essential for intracellular communication and molecular transfer.24 TCs release three types of extracellular vesicles (exosomes, ectosomes and multivesicular cargos) (Figure 3). Evs have been previously described in the myocardium and might be involved in the paracrine effects of cells residing in a normal heart.25 Exosomes, which contain mRNA and can be shuttled from one cell to another, were essential for TCs connected with all types of cells present nearby. Recent studies observed the presence of small exosomes between the TCs and immune cells.26 Predicative function via direct cell contacts and paracrine MVs/exosome release of TCs (Figures 2 and 3). TCs exosomes were found by several groups.27 Our previous studies isolated mice lung TCs exosomes and identified them by ultrastructure and surface protein markers. We demonstrated that the expression level of CD63 is high, while CD9 was low in mice lung TCs-derived exosomes.28
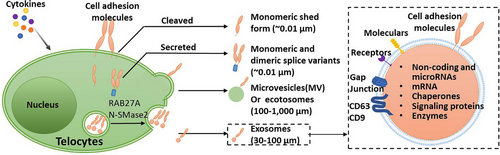
TCs are strongly involved in cell signalling both in normal and pathological processes and behave as nurse cells for stem cell niches.29 A previous study reported that TC-EVs transfer macromolecular signals to adjacent cells to stimulate neovascularization in infarcted myocardium.30 The decrease of TCs in adult hearts may be one of the reasons for the limited cardiac regeneration ability in the elderly.31 TC-derived exosomes attenuate cardiac fibrosis and reduce collagen deposition.32 TCs seem to be able to induce and influence healthy homeostasis and pathological changes in aortic cell/ECM composition. For instance, in case of vascular injury or damage (eg continuous cell stress), smooth muscle cells are modulated (or dedifferentiate) from a mature ‘contractile’ to a less differentiated ‘synthetic’ phenotype.33
TC-derived exosomes could down-regulate the expression of HIF1α, p-AKT and p-mTOR of human bronchial epithelial cells in the culture condition or treatment with lipopolysaccharide (LPS).28 New evidence suggested that calcification-competent EVs from valvular interstitial cells (VICs) are regulators of calcification in diseased aortic valves and atherosclerotic plaques.34 TC-derived exosomes have been demonstrated crucial in maintaining intestinal stem cell function by producing exosomes that contain soluble angiogenic factors, including VEGF-A, KLF-4 and PDGF-A.35 It was revealed that TC-EVs, carrying miR-30b, had inhibited the WNT/beta-catenin pathway, and had impeded osteogenesis and calcium deposition within valvular interstitial cells.36 Although there are no studies using TC-derived exosomes as drugs, TC-derived EVs have been accepted as biomarkers and transferring tools for drugs, vaccines and genes of cardiovascular disease.37 Given the heterogeneous molecular profiles of TCs, it is crucial to understand the specific role of TCs subpopulations regarding signal throughput implementation.
2.3 TCs microRNA-mediated cell communication
microRNAs (miRs) are highly conserved nonprotein-coding RNAs that maintain intracellular homeostasis through negative gene regulation.38 Deregulation of miRs is extensively reported in the progression and prognosis of ARDS and IPF.39 Extracellular vesicles derived from TCs can transfer macromolecular signals such as miRs to neighbouring cells and change their transcriptional activity.40 TCs could produce higher levels of miRNA-21-5p and miR-30b after being stimulated with LPS. TC-EVs could be absorbed by valvular interstitial cells, and miR-30b expression in calcified valvular tissues was notably increased after EV treatment, indicating that TC-EVs were the communication media of miRNAs.41 Yu et al. found a negative association between miR-30 and matrix metalloproteinase (MMP)-9, hinting that it might be a direct target. MiR-30a/c can regulate TM4SF1 and further regulate cell proliferation and stemness.42 miR-21 could form a complex network with targeted genes including MMP-9 and be associated with oxidative stress, inflammation, and fibrosis.43
3 ROLES OF TCs MEDIATED CELL COMMUNICATION IN PULMONARY FIBROSIS OF ARDS
Multiple aberrant pathways interconnect to result in pulmonary fibrosis in a subset of individuals who develop ARDS.44 Important mediators include the dysregulated release of matrix metalloproteinases during the inflammatory phase of ARDS, which causes epithelial and endothelial injury and unchecked fibroproliferation45 (Figure 4 A,B). Canonical profibrotic pathways regulated by TGF-β are important, and there is evidence that vascular dysfunction is a key component of the switch from ARDS to fibrosis, with VEGF and cytokines such as interleukin 6 (IL-6) and TNF-α implicated.46, 47 It remains unclear why certain individuals could recover from such an insult, whereas in others there is a shift to unchecked cellular proliferation with the accumulation of fibroblasts and myofibroblasts and the excessive deposition of collagen alongside other components of the ECM to result in progressive pulmonary fibrosis. Functions and mechanisms of TCs in healthy alveolus and from ARDS to fibroproliferative ARDS were summarized and shown in the pattern diagram (Figure 4).
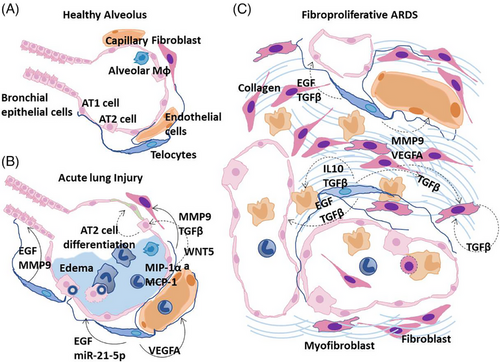
3.1 TCs and alleviation of epithelial injury
3.1.1 Epithelial injury in fibroproliferative ARDS
lung epithelium acts as the first line of defence for inspired air.48 ARDS and pulmonary fibrosis pathogenesis shared the same beginning factor of epithelial cell injury with the secondary fibroproliferative cascade. There are two types of alveolar epithelial cell types which mainly include alveolar type I (AT1) and type II (AT2) epithelial cells.49 AT1 cells account for the vast majority of the surface area of the alveolus, forming the air-blood interface with the underlying interstitium and capillary system.50 The process of trans-differentiation from AT2 to AT1 cells is crucial to ensure a successful re-epithelialization following injury. Thus, AT2 cells are considered the stem cells of the distal lung and are responsible for surfactant production and re-epithelization of the bronchoalveolar epithelium after injury.51 In the progress of ARDS, viral-mediated cell death of the epithelial cells impairs bronchoalveolar epithelial repair, and surfactant production, on the other hand, crosstalk between the alveolar epithelium and other lung cells including fibroblasts and the endothelium, such as increased expression of tissue factor.52 Depletion of the alveolar stem cell population and activation of aberrant repair processes would further lead to pulmonary fibrosis in diseases such as IPF.7
3.1.2 TCs and epithelial regeneration
Tissue regeneration is one of the subtle mechanisms to assure tissue homeostasis. The regenerative ability of adult tissues is achieved by the adult stem cell population resident in niches. Stem cells stay in a quiescent state, and their proliferation or differentiation depends on the change of microenvironment.53-55 TCs were identified in the stem cell niche in the interstitium of lung tissue and intestinal, providing signalling stroma cells which regulated the transformation of intestinal stem cells or AT2 cells into AT1 epithelial lineage.56 TCs contact with stem cells in different organs.57 Reports showed that TCs can stimulate stem cells via paracrine signals.58 For instance, mouse cardiac TCs secrete IL-6, VEGF, macrophage inflammatory protein 1α (MIP-1α), MIP-2 and monocyte chemoattractant protein-1 (MCP-1), while rat TCs secrete more cytokines: IL-2, IL-10, IL-13 and some chemokines (stimulated by IL-6 signalling).59 IL-13 stimulated secretion of VEGF and its receptor (sFlt-1) by oviductal epithelial cells in vivo. Alterations of miRNA expression profile have been reported during the process of transdifferentiating from AT2 to AT1 cells suggesting potential roles of miRNA in the regeneration of the alveolar space following injury.60 Recent reports identified FoxL1-expressing (FoxL1+) subepithelial TCs provide Wnt signaling thus indispensable for intestinal stem cells to survive.61
TCs disappeared in tissue samples affected by pathological processes such as intestinal dysmotility and atrial amyloidosis, suggesting that the loss of these cells correlates with disease and leads to organ dysmotility.56 This indicated that exogenous TCs infusion might be beneficial in organ injury although there are no reports that the number of TCs changed in normal lung tissue and lung diseases.
3.2 TCs in endothelial injury and sprouting angiogenesis
3.2.1 Endothelial injury in fibroproliferative ARDS
Disruption of the endothelium lining the air-blood barrier is recognized as a provoking insult that can initiate ARDS pathogenesis.62 The subsequent pulmonary oedema has been demonstrated to be involved in initiating repair pathways that may become altered and push the lung towards excessive fibroproliferation. Given the substantial impact of severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) infection on endothelial cells, complement deposition, and microthrombosis, including pulmonary capillaries,63 vascular damage is likely more severe in ARDS due to COVID-19 compared with other causes.64
Alveolar oedema is typically resolved after lung injury, but persistent vascular leak is hypothesized to exacerbate the pro-fibrotic environment within the distal lung and has been demonstrated to increase fibrosis in animal models.65 Persistence of fluid within the airspace also distorts the architecture of the alveolus imparting mechanical forces which impair a return to homeostasis.66
3.2.2 TCs and sprouting angiogenesis
Several studies found that TCs could inhibit microvascular endothelial cell apoptosis and promote angiogenesis in myocardial infarction and in chronic inflammation of the uterus and ovaries, such as uterine myoma.67 Recent reports showed that tubal TCs are indirectly involved in local angiogenesis, particularly the CD34+ TCs subtype.68 These TCs dissociated from their perivascular niche, formed a composite structure with fibrin and fibronectin, proliferated and differentiated to be stromal cells during granulation tissue formation.69 Additionally, TCs also express measurable quantities of pro- angiogenic microRNAs (let-7e, 10a, 21, 27b, 100, 126-3p, 130a, 143, 155 and 503).30, 35 The abovementioned indicates the involvement of TCs in neo-angiogenesis.70 TCs function in local angiogenesis was regulated by angiogenic factors, hormones, and paracrine secretion.71 They are positive for estrogen and progesterone, may stimulate the production of VEGF and its receptor by secreted interleukins, and are positive for growth factor receptors (PDGF and VEGF).72 In oviducts from patients with uterine myoma, which have no gross histological changes, the pool of TCs has increased.73 It reflects that TCs might be considered as the line of cells, involved in promoting angiogenesis in inflammation tissues.
3.3 Mechanical stretch and ECM biology
3.3.1 TCs and mechanical stretch
The fibrotic lung is characterized by a highly inhomogeneous mechanical microenvironment, where lung areas with preserved elasticity are contiguous to areas of the rigid lung, resulting in a peculiar mechanical behaviour during inflation, especially in terms of the stress/strain relationship.74 The increased parenchymal stiffness of fibrotic lungs significantly affects respiratory mechanics, making the lung more fragile and prone to non-physiological stress during spontaneous breathing and mechanical ventilation.75 Assisted ventilatory support, one of the important respiratory support measures for ARDS patients, could improve the progression of pulmonary fibrosis.76
Recent research showed that the synthetic exertion and proliferation of TCs in atrium cordis tissue could respond to myocardial hemodynamic overload.77 Moreover, TCs can change their shape, structure and mechanical properties in response to mechanical stress indicating its function under mechanical stretch.78 On the other hand, TCs' cytoskeletal structure actively and closely influences their function, especially their role in intercellular communication in a given environment.79 The biomechanical features of the lung are the result of ECM composition.11 Recently, it was reported that the cell mechanics and kinetics of TCs were quantitatively described through systematic laboratory tests and appropriate numerical simulation to understand the reaction mechanism of TC to surrounding matrix changes and its impact on cell differentiation in regenerative medicine.79
3.3.2 ECM biology
In severe cases of ARDS in which the proliferative phase is more exaggerated, decreased compliance of the parenchymal tissue may further activate the fibrotic response leading to the dramatic fibrosis that occurs in a subset of patients. Activated fibroblast is the main resource of ECM.64 TCs make heterocellular contacts with fibroblasts and myofibroblasts and indirectly impact the activity of these cells.80 TCs are positive for steroid hormone receptors, which may impact the mechanism of protein production. Elastin and glycoprotein such as fibronectin are both essential in driving cells toward a profibrotic phenotype with the induction of myofibroblast differentiation through TGF-β1 pathway amplification.81 TCs decreased TGF-β1 levels in serum, suppressed Smad2/3 phosphorylation, and increased the expression of hepatocyte growth factor (HGF) in rat kidney tissue.82 In addition, ECM remodelling is associated with specific MMPs/Tissue inhibitors of metalloproteinases (MMPs/TIMPs) balance.83 Thus, excessive factor expression/secretion of TCs could lead to pathological ECM remodelling.
3.3.3 Cell adhesion molecules of TCs
Interactions between cell adhesion molecules and underlying ECM have also been a productive area of investigation in IPF biology. Integrins, which bind the basal surface of cells to the ECM, have been sought as possible therapeutic targets for their known secondary function in activating latent transforming growth factor-β bound within the ECM.84 The SARS-CoV-2 virus is able to bind integrins via a conserved motif near its receptor binding domain. Although the functional consequence of viral particles binding to membrane-bound integrins is not entirely clear, it has been demonstrated to increase viral entry into cells.85-87 One integrin in particular, αvβ6, appears to have a relatively high affinity for the SARS-CoV-2 virus and has been directly implicated in IPF pathogenesis, again suggesting a link between these seemingly distinct processes.88
4 THE EFFECT OF HETEROCELLULAR CONTACTS OR PATHOLOGY ENVIRONMENT ON TCs
We also want to briefly discuss the regulation of components in fibroproliferative ARDS pathology environment on TCs function.31 TCs are characterized by high sensitivity to hypoxia and probably the development of local inflammation leads to TCs damage through angiogenesis and imbalance in pro- and anti-angiogenetic factors.89 TCs are identified in close vicinity to blood vessels, they are sensitive to angiogenic factors (PDGF and VEGF) and ischemia, and the number of TCs declined or disappeared in fibrosis.90 TCs own genetic and microRNA profiles reflected their sensitivity to hypoxia as well. TCs damage or decrease in number activates tissue repairing mechanisms. In tissue samples from patients with psoriatic lesions, TCs have degenerative features (apoptosis, membrane disintegration, cytoplasm fragmentation, and nuclear extrusion).91 The development of fibrosis might be the most important implication of TCs deficiency in the lungs.92 TCs would be injured when normal architecture was detrimental as interstitium cartilaginous and osseous metaplasia in the later stages of myocardial infarction.93 The evidence of TCs heterocellular interaction was provided in recent studies. Inflammation factors induced TCs to provide HGF to macrophages and transfer from M1 to M1/M2 state.94 TCs mediated cell-cell communication with multiple cell types in the tissue microenvironment was emphasized thus contributing to tissue renewal, repair and the stability of the microenvironment.95 It has been shown that TCs co-cultured with Peritoneal macrophages could inhibit apoptosis.96
5 ANTI-FIBROTIC-TARGETS OF TCs FOR FIBROSIS TREATMENT
Growing evidence showed that the IFN-γ, TGF-β, WNT and PI3K/AKT signalling pathways play a key role in ARDS and lung fibrogenesis via the regulation of cell survival, apoptosis and regeneration.97, 98 Moreover, TCs were widely implicated in the modulation of these signalling pathways that might be recommended as an important target for discovering drugs for lung fibrogenesis.
5.1 TCs prevent fibroproliferative ARDS by regulating IFN-γ levels in damaged tissues
Early antifibrotic studies focused on key antiviral proteins, such as IFN-β and IFN-γ.99 Cytokine storm, which is composed of IFN-γ, IL-6, etc. cytokines, might be the cause of severe complications of COVID-19.100 In addition, the exogenous or endogenous IFN-γ produced by early treatment applications may trigger pulmonary vascular disease.101 Concentration of circulating IFN-γ and CXCL10 were increased in patients with severe COVID-19, this situation also occurs in the lung tissue of acute lung injury animal models.102
5.2 TGF-β levels modulation of TCs in fibroproliferative ARDS
Another target of anti-fibrosis therapy is TGF- β pathways.103 TGF-β plays multiple homeostatic roles in the regulation of inflammation, proliferation, differentiation and wound healing of various tissues.104 TGF-β can induce SMAD-dependent (canonical feedback) or SMAD-independent (non-canonical feedback) to moderate non-canonical signalling responses through the WNT, PI3K/AKT, Mitogen-activated protein kinase (MAPK) pathways.105-107 During inflammation, TGF-β has two important functions, including the promotion of wound healing and suppression of inflammation.108 However, TGF-β stimulates the activation and proliferation of fibroblasts, which result in ECM deposition.109 Therefore, various strategies with TGF-β, TGF-β ligands or αvβ6 integrin to prevent binding to receptors or activation of latent TGF-β respectively had been used for inducing fibrosis in both clinical trial and experimental models of fibrotic diseases such as pulmonary fibrosis.110 Mice lung TCs could express and release higher levels of TGF-β when stimulated by LPS or OVA. In addition, the TCs subtype in the subepithelial stroma could provide Wnt/β-catenin signalling to cooperate with the TGF-β/BMP pathway to modulate a tissue microenvironment.111
5.3 Wnt signalling provided by TCs in fibroproliferative ARDS
The Wnt signalling pathway is considered the key signalling pathway in the process of ARDS and lung fibrosis.112 Recent studies have shown that TCs provide Wnt family ligands and related proteins through the formation of a subepithelial network, which could support the renewal of adjacent cells and tissues in the intestine.113 It was reported that TCs were the inhibitors of Wnts along the length of their intestinal crypts, and the higher expression levels of Wnt at the bottom of the crypts can activate Wilt signal conduction in stem cells.114 TCs may play an important role as the connection unit for direct communication with other types of units. Previous studies have proved that the paracrine effect of TCs can enhance the proliferation, adhesion, and motility of ESC in vitro through the ERK pathway.115 TCs could modulate cell-cell communication via the Wnt signalling pathway and further promote regeneration.116 A unique secretory cell population in human respiratory airways was identified in proximal upper airways, which termed RAS cells, could serve as a previously undescribed distal lung progenitor population by differentiating into AT2 cells rapidly, and this process was controlled by Notch and Wnt signaling.74, 117
6 CONCLUSION
In the past years, the sequelae of ARDS gradually appeared, including fibroproliferative ARDS. Although relevant medical care has been optimized and standardized, morbidity and mortality of fibroproliferative ARDS are still high. Fibroproliferative ARDS has become a long-term chronic disease, and acute lung injury is a triggering factor. Epithelial injury, endothelial integrity deterioration, pulmonary interstitial structural damage, and lung tissue regeneration disorders play comprehensive roles in the progression of ARDS. TCs regulate the functions of multi-type cells in lung tissue through cell-cell communication, thus maintaining the stability of the microenvironment. Therefore, the exploration of TCs-dominated cell-cell communication in pathological conditions is an attractive research direction for developing new cell therapeutics.
7 PERSPECTIVES
Recent and current research mainly focused on preclinical and clinical studies of regenerative therapy based on skeletal muscle fibroblasts or bone marrow stem cells.112 However, the predicted outcome is still difficult to achieve. Therefore, considering the currently published data on TCs functions in ARDS and fibrosis and its collaboration with structural elements, it is necessary to evaluate TCs' intention in lung inflammation alleviation and regeneration promotion, in order to prevent or treat fibroproliferative ARDS in the early stage. Both classic stem cell therapy and TCs-MSCs combination therapy should be considered more promising in the therapeutic process of lung regeneration/repair.
Improving the target exogenous cell proportion motive to the damaged area is one of the prominent issues faced in cell therapy. TCs support the transplantation of stem cells to target lung tissue and promote the survival of transplanted cells. Additional attractive solutions are pretreatment TCs with paracrine factors, tissue guidance, blood vessel scaffolds and/or neural inputs, to stimulate the cooperation between TCs and other cell types. Therefore, the availability and plasticity of TCs have greater advantages, which may be a missing link or new target in acute and chronic lung disease therapy, including fibroproliferative ARDS.
AUTHOR CONTRIBUTIONS
Yonghua Zheng, Dongli Song and Songshan Cai write the manuscript, Zongfeng Zhao collect the references, Xiangdong Wang and Lihua Dai design the review, and Dongli Song revise the manuscript and draw the figures.
ACKNOWLEDGEMENTS
The work was supported by the Science and Technology Commission of Shanghai Municipality (21140902601, 21140902600 and 21ZR1412800).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




