Chances and challenges in intranasal administration delivery for brain disease treatment
Abstract
In order to overcome the formidable challenges posed by the intricate physiological barriers of the brain, the employment of intranasal administration (INA) has emerged as an unconventional method for drug delivery, offering distinct advantages such as non-invasiveness and enhanced pharmacokinetic characteristics within the brain. Primarily exploiting the distinct conduit offered by the olfactory and/or trigeminal nerve systems, the INA route effectively delivers therapeutic agents. With introducing appropriate improvements to the drug formulation, such as the incorporation of nanocarriers, the efficacious delivery via the INA approach has gained considerable traction for the treatment of neurological disorders. This concise review highlights the notable progress in INA delivery and explores the potential therapeutic modalities inherent in this promising paradigm.
1 THE WAY TO THE BRAIN
The intricate workings of the brain rely on a carefully balanced homeostatic environment that governs the ionic composition of the interstitial fluid. This delicate regulation is maintained through the concerted efforts of various protective structures within the central nervous system (CNS), encompassing the skull, meninges, blood-brain barrier (BBB) and blood-cerebrospinal fluid (CSF) barrier (Figure 1).1-3 These barriers not only serve as the crucial purpose of safeguarding the brain but also impose significant limitations on the effective delivery of therapeutic drugs to the brain parenchyma, thereby impeding the treatment of brain disorders. In light of such a challenge, intranasal administration (INA) has emerged as a novel avenue for drug entry into the brain initially pioneered by William H. Frey in the 1990s. Nowadays, INA has since been applied in a diverse array of drug delivery approaches, encompassing chemical drugs, liposomes, oncolytic viruses for tumour vaccines and other relevant substrates.4-6
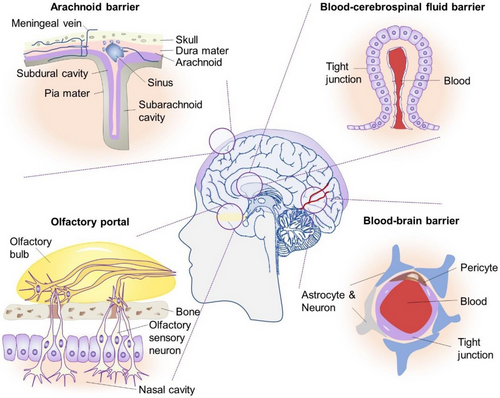
2 MAIN PHYSIOLOGICAL PATHWAYS OF INA IN THE BRAIN
The INA delivery system plays an important role in bypassing the brain barriers. Studies have indicated that INA primarily relies on two routes to transport drugs into the brain: (1) Neural pathways: This route involves the drug entering the brain through the olfactory nerve and trigeminal nerve, which are located beneath the nasal mucosa. The olfactory nerve is directly connected to the olfactory bulb, while the trigeminal nerve connects to the brainstem. By utilizing these neural pathways, drugs can bypass the BBB and directly reach the brain. (2) Vascular route: In this route, the drug initially enters the systemic circulation by crossing the capillaries in the nasal mucosa. From there, it can then cross the BBB through various mechanisms to reach the brain. This route involves the drug traveling through the bloodstream to gain access to the brain. Both of these routes play a significant role in facilitating the delivery of drugs to the brain through the intranasal administration method. By utilizing the INA delivery system, drugs can potentially achieve higher concentrations in the brain compared to other administration methods, offering a promising approach for targeted drug delivery to the central nervous system7, 8(Figure 2).
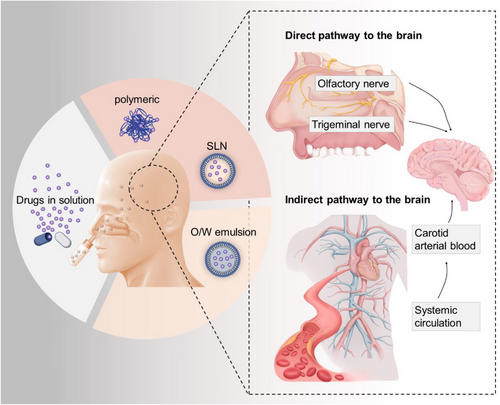
Indeed, the INA route has several appealing aspects when it comes to drug delivery. Some of these advantages include: (1) Direct absorption into the brain: The nasal mucosa is highly vascularized, allowing for direct absorption of drugs into the brain. This bypasses the need for drugs to go through the gastrointestinal tract or undergo first-pass metabolism in the liver, resulting in a faster onset of action. (2) Reduced dose requirements: By avoiding first-pass metabolism and pharmacokinetic variations associated with oral administration, lower doses of medications may be required to achieve the desired therapeutic effect. This can potentially minimize side effects and improve patient compliance.8, 11
However, INA does have certain limitations that can affect the bioavailability of drugs in the brain: (1) Absorption capacity of nasal mucosa: The nasal mucosa has a limited surface area, and its absorption capacity can restrict the amount of drug that can be absorbed. This limitation may require optimization of drug formulations to enhance absorption and increase bioavailability. (2) High molecular weight of drugs: Drugs with high molecular weights may face challenges in crossing the nasal mucosa and reaching the brain. Modification of drug formulations or the use of drug delivery systems, such as nanocarriers, can help overcome this limitation by improving drug stability and penetration. (3) Biodegradation of drugs: The nasal mucosa contains enzymes that can degrade certain drugs, reducing their bioavailability. Modifications to drug formulations, such as the use of prodrugs or enzyme inhibitors, can help improve drug stability and prolong their presence in the nasal mucosa.9, 10
To fully realize the potential applications of INA, utilizing appropriate drug modifications and formulations such as nanocarriers made from biomaterials to enhance drug stability, prolong drug releasing and improve drug penetration into the brain are necessary. These modifications aim to address the limitations associated with the INA route and maximize the therapeutic benefits of intranasal drug delivery.9-11
3 PRECLINICAL AND CLINICAL APPLICATION OF INA DELIVERY SYSTEM TO TREAT NEUROLOGICAL DISORDERS
Currently, INA has found extensive use in the treatment of various neurological conditions (Figure 3), including: (1) Neurodegenerative diseases: INA has been investigated for the treatment of neurodegenerative disorders such as Parkinson's disease and Alzheimer's disease. Studies in animal models have shown promising results using INA for the delivery of insulin, nanoparticles, in situ gelation systems, and peptides to improve the symptoms associated with these diseases.5, 12-15 (2) CNS Injuries (e.g., Stroke): INA has also been explored for the treatment of CNS injuries, particularly stroke. (3) Brain tumours: INA has been studied as a delivery method for treating brain tumours. It offers a non-invasive approach to deliver anticancer drugs directly to the brain, bypassing the blood-brain barrier and minimizing systemic side effects. (4) mental disorders: INA has shown promise in the treatment of mental disorders. Research has focused on using INA to deliver medications, such as antipsychotics and antidepressants, for improved drug delivery to the brain and enhanced therapeutic outcomes. While preclinical studies have demonstrated the positive effects of INA in animal models, translational studies in humans are relatively limited. One notable double-blind clinical trial involved 289 adults who were randomized to receive nasal insulin delivery or a placebo. The study concluded that there were no observed cognitive or functional benefits from nasal insulin treatment compared to the placebo group.16 This highlights the need for further research and larger clinical trials to determine the efficacy of INA in treating neurological conditions in humans.
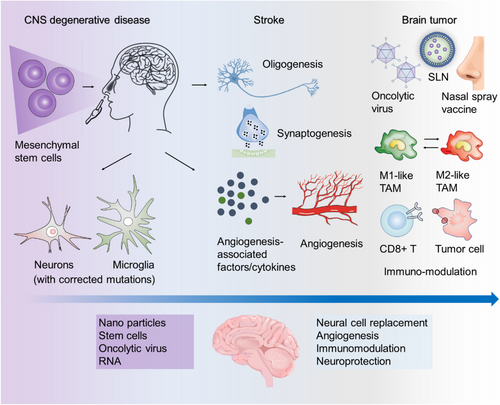
Notably, INA of stem cells is emerging as a promising therapy for treating CNS injuries, owing to the advantages of stem cells in functional repair of cerebrovascular diseases. It has been demonstrated that peripheral vascular endothelial progenitor cells have the ability to repair damaged vascular endothelial cells, promoting vascular regeneration in vivo.17, 18 Moreover, clinical studies have provided evidence that INA of mesenchymal stromal cells in neonates following perinatal arterial ischemic stroke is a feasible approach, with no significant adverse events observed in patients followed up to 3 months of age. Nevertheless, further clinical trials are necessary to fully harness the potential of stem cells for CNS injury treatment. Therefore, achieving outputs in this area will contribute to advancing the clinical utilization of stem cells in the field of CNS injury treatment.19-21
Brain tumours, particularly glioma, are a significant category of brain diseases that pose a significant challenge in terms of treatment. The low bioavailability of most drugs due to their limited ability to penetrate the BBB necessitates the development of new drug treatment systems.22 INA in combination with nano-delivery systems has emerged as a promising approach to address these issues through significantly enhancing drug efficacy. By utilizing nanocarriers, the nasal residence time of drugs can be extended, resulting in substantial improvements in pharmacokinetics and biodistribution within the intranasal nano-delivery system.23, 24 Various systems are currently employed for nasal drug delivery, including vesicular systems such as liposomes, emulsion systems like nanoemulsions and solid lipid nanoparticles, and micellar systems.25-27 These systems facilitate the efficient delivery of drugs to the brain through INA. Additionally, recent advancements in cancer immunotherapy have focused on the INA of tumour vaccines for glioma treatment. This includes the use of oncolytic viruses, short peptides, and synthesized antibodies.28
Besides, in order to promote these systems further into clinical practice, tracking technologies with high spatiotemporal resolution are in demand. Tracking material functionalized with optical and biological properties, that is, gold nanorods with fluorescent probes, could provide real-time spatiotemporal information for brain uptake pattern and distribution after INA. Recent developments in imaging technologies such as optical imaging, inductively coupled plasma mass spectrometry (ICP-MS), and autoradiography both show benefits and limitations. For example, ICP-MS could provide sensitive quantification of gold content measurement, while it is labor intensive without offering intracellular information.24
4 CONCLUSION AND PERSPECTIVES
The successful clinical application of therapeutic drugs targeting specific CNS disorders relies on an effective drug delivery pathway with optimal bioavailability in the CNS. The INA delivery system offers a direct and efficient non-invasive route for delivering drugs to the brain. Recent advancements have demonstrated the potential of the INA delivery system in the treatment of various conditions, including migraines, cognitive dysfunction disorders, cerebral hemorrhages and tumours. However, despite notable progress in recent years, there is still a substantial gap between these achievements and their clinical translation (Table 1). Several key aspects warrant further research in this field:
| Clinical trial | Intervention | Study phase | Goal | Outcome measures | Year of initiation |
|---|---|---|---|---|---|
| NCT04091503 |
|
Phase I |
|
|
2019 |
| NCT00748956 |
|
Phase II |
|
|
2010 |
| NCT03081416 |
|
Phase III |
|
|
2016 |
| NCT02414503 |
|
Phase I & II |
|
|
2015 |
| NCT01533519 |
|
Phase I |
|
|
2012 |
| NCT00422981 |
|
Phase II |
|
|
2007 |
(1) Animal models that accurately reflect clinical evaluation: Suitable animal models are useful for characterizing the nasal absorption and pharmacokinetic nature of INA. However, due to the anatomical and physiological differences among species (Table 2), there remains a gap between animal models and human clinical practice.29, 30 Besides, although improvement in motor features after INA delivery can be well assessed in animal models, the evaluation of non-motor functions such as mood, sleep conditions and cognition remains limited and requires further development.31, 32
| Species | Mean nasal volume (mL) | Mean nasal length (cm) | Mean nasal surface area (cm2) | Conchae structure | Expected clearance half-life (min) |
|---|---|---|---|---|---|
| Human | 20 | 7.5 | 160 | Single scroll | 15 |
| Monkey | 8 | 10 | 62 | Single scroll | 10 |
| Guinea pig | 0.9 | 3.4 | 27 | Double scroll | 7 |
| Dog | 20 | 10 | 221 | Branching | 20 |
| Rabbit | 6 | 5.2 | 61 | Branching | 10 |
| Rat | 0.4 | 2.3 | 14 | Double scroll | 5 |
| Mouse | 0.03 | 0.5 | 2.8 | Double scroll | 1 |
(2) Multimodal real-time tracing system for precise assessment of INA delivery efficiency: Currently, the quantification of INA delivery efficiency primarily relies on mathematical modeling and tomographic slices. To advance our understanding, additional multimodal imaging technologies are needed for studying brain cell uptake and the real-time localization of drugs.33-36
(3) In-depth investigation of interactions between nano-delivery systems, drug formulation, and neural pathways: The precise mechanism by which drugs enter the brain through INA delivery systems remains unclear. Combining in-silico modeling of the intranasal environment with experimental studies on drug-nanocarrier interactions and their interactions with cells can provide valuable insights for the rational design of drug delivery systems.37
The precise mechanism by which drugs enter the brain through INA delivery systems is still not fully understood. A comprehensive understanding of this process can be achieved through the application of in silico modeling techniques to simulate the intranasal environment. Additionally, conducting experimental studies to investigate the interactions among the drug, nanocarriers, and cells involved in the delivery process is crucial. These investigations can provide valuable insights and contribute to the rational design of drug delivery systems for INA, ultimately enhancing their efficacy and clinical applicability.38-41
In summary, the INA approach holds promise as a therapeutic strategy to overcome the limitations imposed by the BBB. To fully realize the potential of INA in the treatment of brain diseases, further interdisciplinary collaboration and research efforts are needed. By working together, scientists and healthcare professionals can optimize the INA delivery system, develop novel drug formulations, and improve our understanding of the underlying mechanisms. Addressing these research areas will contribute to enhancing our understanding of the INA delivery system, optimizing drug delivery to the CNS, and advancing the development of effective therapies for CNS disorders. This collaborative approach will pave the way for more effective and targeted treatments for a wide range of brain diseases in the future.
ACKNOWLEDGEMENTS
This work was funded by the National Natural Science Foundation of China, grant number: 82004081, 52073145, and 82200043 and the National Natural Science Foundation of Nanjing University of Chinese Medicine, grant number: NZY82004081.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Please contact the corresponding author for all data requests.




