Impact of prior coronavirus disease 2019 infection in females on assisted reproductive outcomes: A systematic review with meta-analysis
Yiqi Liu, Shen Chen, Mengyi Chen and Chutian Xing contributed equally to this work.
Abstract
Objectives
To investigate the relationship between prior coronavirus disease 2019 (COVID-19) infection in females and subsequent treatment outcomes of assisted reproductive technology (ART).
Methods
A systematic literature review was carried out up to 16 December 2022, in PubMed, Web of Science, EMBASE, and Cochrane Library. Random-effect models were adopted to estimate the pooled effects as mean differences (MDs) or odds ratios (ORs). I2 statistic and Egger's test were applied to assess heterogeneity and publication bias, respectively.
Results
After screening 1480 records, 15 cohort studies totalling 1905 cycles were included in this meta-analysis. In a comparison of previously COVID-19-infected versus uninfected women, no significant differences were observed in the primary outcomes of the retrieved oocytes number (MD = 0.06; 95% confidence interval [CI]: -0.75–0.88; I2 = 0) and clinical pregnancy rate (OR = 0.96; 95% CI: 0.74–1.24; I2 = 0). Pooled analyses of other predefined outcomes, which encompassed four cycle characteristics, six laboratory indicators and four pregnancy results, also showed no adverse effects of prior COVID-19 infection. Most outcomes remained consistent after further sensitivity and subgroup analyses, and no significant publication bias was observed.
Conclusions
Our work provides the first systematic evidence that COVID-19 infection history in females may have no measurable detrimental impact on the subsequent ART cycle. More data are needed to assess the live birth outcome and the optimal time interval from infection to assisted reproduction.
1 INTRODUCTION
Since late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally at a rapid speed, which is the causative agent of coronavirus disease 2019 (COVID-19).1 As of October 18, 2023, there have been more than 770 million cumulative confirmed cases with approximately 7.0 million deaths according to the World Health Organization,2 posing a serious threat to public health.
Previous studies have demonstrated that the SARS-CoV-2 spike (S) protein could bind to the angiotensin-converting enzyme 2 (ACE2) with a high affinity located on the cell surface.3-5 Transmembrane protease serine 2 (TMPRSS2), on the other hand, could cleave the S protein and play an essential role in mediating viral entry into cells.3, 6 Therefore, the expression and distribution of ACE2 and TMPRSS2 in the body largely determines organ susceptibility to the invasion of SARS-CoV-2. Besides, single-cell RNA sequencing has proved the presence of these two entry factors in the ovary and uterus apart from the lung, indicating the possibility of the virus directly invading the female reproductive system.7, 8 Moreover, SARS-CoV-2 was found in the vaginal swabs of females infected by COVID-19.9-11 Even in the absence of direct infection, cytokine storms induced by COVID-19 could also adversely alter endometrial cell gene expression.12
In this regard, clinical attention is being emergingly paid to the post-infection impact on female fertility as well as treatment outcomes of assisted reproductive technology (ART).13 The first cohort by Orvieto et al.14 found a reduced percentage of top-quality embryos in women recovered from COVID-19. However, this finding was not observed in a subsequent study by Bentov et al.15 The inconsistent conclusions of individual studies, together with differences in included population, sample size and follow-up period, imply the need for pooled evidence.
The goal of this systematic review was to comprehensively evaluate the impact of female COVID-19 infection history on ART performance, covering cycle characteristics, laboratory indicators and pregnancy results.
2 METHODS
The current study was conducted in conformity with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement16 and the Meta-Analysis of Observational Studies in Epidemiology guideline.17 The protocol has been submitted for registration in the International Platform for Registered Systematic Review and Meta-analysis Protocols (No. INPLASY202330106), available at: https://inplasy.com.
2.1 Search strategy
To identify studies meeting the inclusion criteria, a literature search was conducted across PubMed, Web of Science, Excerpt Medica Database (EMBASE) and Cochrane Library. The search period lasted from inception to December 16, 2022, and no language restrictions were applied. The keywords combination we adopted was as follows: (‘COVID-19′ OR ‘SARS-CoV-2′) AND (‘reproductive techniques, assisted’) OR (‘sperm injections, intracytoplasmic’) OR (‘fertilization in vitro’) OR (‘embryo transfer’) OR (‘embryo implantation’). Furthermore, we thoroughly examined the bibliographies of the selected paper along with the conference proceedings of ESHRE and ASRM to ensure that potentially relevant records were not omitted.
2.2 Study selection
The eligibility of the searched literature was evaluated according to the inclusion criteria as follows: 1) the study was observationally designed, such as cohort; 2) the participants were infertile women who underwent ART treatment cycles; 3) the exposed group had been infected with COVID-19 in the past and now recovered, while previously uninfected individuals served as the control group; 4) the outcomes encompassed any predefined information concerning cycle characteristics, laboratory indicators, and pregnancy results. The cycle characteristics contained data on the duration of stimulation, the total dose of gonadotropin, the peak level of estradiol, and the thickness of the endometrium. The laboratory parameters comprised the number of retrieved oocytes, the number and rate of mature oocytes, the number and rate of fertilized oocytes, as well as the number and rate of good-quality embryos. The pregnancy indicators contained the rate of embryo implantation, the rate of early miscarriage, as well as biochemical, clinical and ongoing pregnancy rates.
Studies were excluded if they: 1) were cell or animal experiments, case reports or series, reviews, editorials, communications, or comments; 2) lacked original outcome data or a control group; 3) were only abstracted without full data; 4) were duplication publications in conference; 5) investigated the impact of COVID-19 infection in males; or 6) studied the effect of COVID-19 pandemics or current COVID-19 infection. Studies with larger sample sizes were given priority when containing overlapping data of the same cohort.
A two-step approach was employed for the identification of studies. Firstly, the titles and/or abstracts of the paper were examined by two independent reviewers (Yiqi Liu and Shen Chen) based on the selection criteria described above. Then, the literature was reviewed in its entirety to ensure its eligibility for inclusion. Any disagreement that arose during the selection process was discussed with the third author (Mengyi Chen) until an agreement was reached.
2.3 Data extraction and study quality assessment
Data extraction was completed by two reviewers (Yiqi Liu and Shen Chen) independently and recorded into the pre-designed Excel form in a standardized manner. To avoid potential errors, we also conducted a reciprocal check with a third reviewer (Mengyi Chen), and the inconsistency was resolved by consulting with the corresponding author (Jialyu Huang). The information gathered was as follows: name of the first author, publication year, country of study, design and period of study, sample size, participants’ age, diagnosis of SARS-CoV-2 infection, disease severity, the time interval from infection to treatment, embryo transfer (ET) strategy, and targeted outcome measures.
Given the final inclusion of cohort studies, the methodological quality was evaluated via the Newcastle-Ottawa Scale (NOS).18 Three main dimensions include cohort selection, comparability, and outcome evaluation, which could be further specified into eight aspects including the representativeness of the exposed group, selection of the control group, determination of exposure, lack of outcome at the outset, comparability of the design or analysis, evaluation of outcome, and the duration and sufficiency of follow-up. Based on the total score, studies were classified as low (0–3), medium4-6 and high7-9 quality. Again, any disputes arising from this process were settled through consultation with the corresponding author (Qiongfang Wu).
2.4 Statistical analysis
Review Manager (version 5.4; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020) was employed to perform data synthesis. Post-matching data had priority for analysis in all included studies. For continuous outcomes, data were pooled as mean differences (MDs), while odds ratios (ORs) were adopted to depict pooled effects for dichotomous data. According to Luo et al.19 and Wan et al.,20 when studies were reported with the complete or partial five-number summary, the mean and standard deviation of the sample were computed from medians, ranges, or interquartile ranges (https://www.math.hkbu.edu.hk/∼tongt/papers/median2mean.html). Since the validity of the heterogeneity test might be affected by a small sample size, the random-effects model was adopted for synthesis even if low heterogeneity was detected in included studies.21
We estimated the heterogeneity across studies via Cochran's Q test, and the inconsistency index (I2) < 50% was considered to indicate low heterogeneity.22 Subgroup analyses were conducted for the two primary outcomes of the retrieved oocyte number and clinical pregnancy rate according to the sample size (≥ or < 100) as well as the study design (retrospective or prospective). Additionally, sensitivity analysis was used to estimate the leverage of individual studies and robustness of the overall results, by sequentially excluding one study at a time. Egger's test was completed through Stata (version 16.0; StataCorp LLC) to evaluate the publication bias. In Egger's test, p < .10 was considered to be of statistical significance,23 while p < .05 was adopted in any other analyses.
3 RESULTS
3.1 Study selection and characteristics
Initially, a total of 1482 studies were identified, with 1480 records from database search and an additional two records from other sources by manual reviewing. After removing duplicates, 846 records remained. Subsequently, 804 records were excluded by title and/or abstract filtering, followed by 29 records through full-text assessment. Finally, a total of 15 studies were included in this systemic review and meta-analysis (Figure 1).14, 15, 24-36
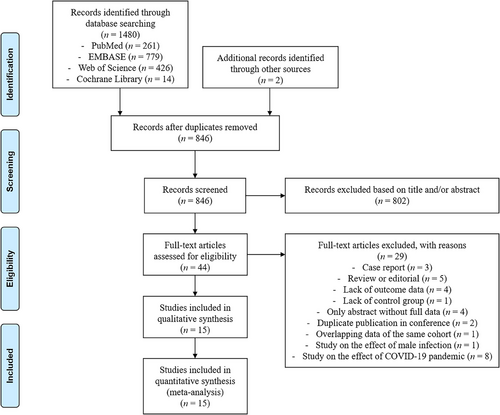
The main characteristics of the included studies are enumerated in Table 1. These studies were distributed in eight countries including China, Israel, the USA, Argentina, Italy, Spain, Russia and Jordan, and all were published between 2021 and 2022. Most of these studies (n = 10) employed a retrospective cohort design14, 25-27, 29, 30, 32-35 and five were prospective cohorts.15, 24, 28, 31, 36 All studies were single-centre except for the studies by Youngster et al.34 and Albeitawi et al.,26 whose participants were enrolled from two centres. The sample size of each study ranged between 7 and 452. The infection of SARS-CoV-2 was diagnosed in four studies by polymerase chain reaction test,14, 24, 33, 35 five by serum antibodies,27, 28, 30, 31, 36 and three using both.15, 29, 32 The remaining studies did not mention the details of diagnosis. The severity of COVID-19 was investigated in seven studies, among which three were asymptomatic or mild,29, 30, 32 two were mild,27, 35 one was mild or severe28 and one was mild or moderate.36 Most studies (n = 11) considered the interval between COVID-19 and the initiation of the post-infection cycle, except those conducted by Morris et al.,30 Odeh-Natour et al.,31 Chico-Sordo et al.,28 and Albeitawi et al.26 In terms of ET strategy reported in 10 studies, five used fresh ET,26, 31, 32, 34, 36 three used frozen-thawed ET25, 30, 33 and two used both.24, 35 Overall, the literature included in this review had high methodological quality, as indicated by the mean NOS score of 8 (all ≥ 7) (Table S1).
| Study | Country | Cohort design | Period | Sample size | Age (years) | Diagnosis of SARS-CoV-2 infection | Severity | Interval to treatment | Embryo transfer |
|---|---|---|---|---|---|---|---|---|---|
| Orvieto et al., 2021 | Israel | Retrospective, single-centre, self-controlled | NA | 7* |
C: 34.0 ± 7.1 I: 34.5 ± 7.4 |
PCR test | NA | 46.4 ± 29.3 days (range 8−92) | – |
| Wang et al., 2021 | China | Retrospective, single-centre | May 2020 to February 2021 | 260 (unmatched: 4043) |
C: 32.0 ± 4.5 I: 31.4 ± 3.8 |
PCR test + serum antibody | Asymptomatic or mild | ≥4 months | Fresh |
| Morris et al., 2021 | USA | Retrospective, single-centre | January 2021 to May 2021 | 108* |
C: mean 34.6 I: mean 33.1 |
Serum antibody | Asymptomatic or mild | NA | Frozen |
| Bentov et al., 2021 | Israel | Prospective, single-center | February 2021 to March 2021 | 23* |
C: 32.5 ± 5.3 I: 34.1 ± 4.7 |
PCR test + serum antibody | NA | 98.1 ± 45.5 days (range 48−169) | – |
| Herrero et al., 2022 | Argentina | Retrospective, single-centre | November 2020 to April 2021 | 80 |
C: 33.1 ± 3.5 I: 33.4 ± 6.9 |
PCR test + serum antibody | Asymptomatic or mild | mean: 4.5 months (range 2−9) | – |
| Aizer et al., 2022 | Israel | Retrospective, single-centre | January 2021 to August 2021 | 452* |
C: 28.9 ± 4.5 I: 30.9 ± 4.0 |
NA | NA | 153 ± 93 days | Frozen |
| Odeh-Natour et al., 2022 | Israel | Prospective, single-center | March 2021 to May 2021 | 27* |
C: 35.7 ± 7.0 I: 31.9 ± 6.4 |
Serum antibody | NA | NA | Fresh |
| Castiglione Morelli et al., 2022 | Italy | Retrospective, single-center | March 2021 to September 2021 | 14* |
C: 36.2 ± 4.2 I: 37.4 ± 5.9 |
Serum antibody | Mild | mean: 7 months (range 4−11) | – |
| Youngster et al., 2022a | Israel | Retrospective, two-center | October 2020 to June 2021 | 242 |
C: 33.2 ± 5.3 I: 33.3 ± 5.4 |
NA | NA | 84.5 ± 78.0 days (range 8−348) | Fresh |
| Youngster et al., 2022b | Israel | Retrospective, single-centre | January 2021 to June 2021 | 82 |
C: 31.6 ± 5.1 I: 31.6 ± 5.2 |
PCR test | NA | 59.7 ± 68.3 days (range 1−274) | Frozen |
| Kabalkin et al., 2022 | Israel | Retrospective, single-centre | March 2020 to April 2021 | 120 |
C: mean 34.8 I: mean 31.9 |
PCR test | Mild | mean: 1.75 months (range 0.5−3) | Fresh and Frozen |
| Adler Lazarovits et al., 2022 | Israel | Prospective, single-centre | October 2021 to November 2021 | 34* |
C: 34.8 ± 5.6 I: 29.9 ± 5.6 |
PCR test | NA | 209.6 ± 85.1 days | Fresh and Frozen |
| Chico-Sordo et al., 2022 | Spain | Prospective, single-centre | October 2020 to May 2021 | 65 |
C: 35.3 ± 5.8 I: 35.8 ± 5.9 |
Serum antibody | Mild or severe | NA | – |
| Dolgushina et al., 2022 | Russia | Prospective, single-centre | NA | 240 |
C: 33.3 ± 4.5 I: 34.0 ± 4.5 |
Serum antibody | Mild or moderate | ≤12 months | Fresh |
| Albeitawi et al., 2022 | Jordan | Retrospective, two-centre | September 2021 to November 2021 | 151 |
C: 34.0 ± 6.9 I: 32.8 ± 5.7 |
NA | NA | NA | Fresh |
- * Only infected (I) and control (C) women were included in the analysis, with the exclusion of vaccinated and male patients.
- Abbreviations: NA, not available; PCR: polymerase chain reaction.; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
3.2 Meta-analysis of cycle characteristics
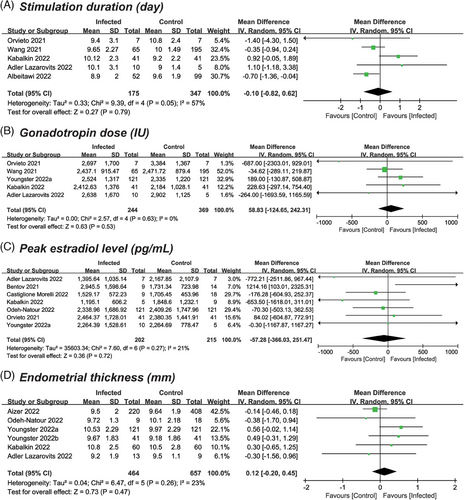
The quantity of included studies and participants varied in reporting cycle characteristics, with five (n = 572) providing data on stimulation duration,14, 24, 26, 32, 35 five (n = 663) on gonadotropin dose,14, 24, 32, 34, 35 seven (n = 467) on peak estradiol level14, 15, 24, 27, 31, 34, 35 and six (n = 957) on endometrial thickness.24, 25, 31, 33-35 As shown in Figure 2, the results revealed that none of the characteristics were significantly affected. The pooled MDs for the comparison of previously infected versus uninfected cycles were 0.10 days (95% confidence interval [CI]: −0.82–0.62; p = .79; I2 = 57%) for duration of stimulation, 58.83 IU (95% CI: −124.65–242.31; p = .53; I2 = 0) for total dose of gonadotropin, −57.28 pg/mL (95% CI: −366.03–251.47; p = .72; I2 = 21%) for peak level of estradiol, and 0.12 mm (95% CI: −0.20–0.45; p = .47; I2 = 23%) for endometrial thickness.
3.3 Meta-analysis of laboratory parameters
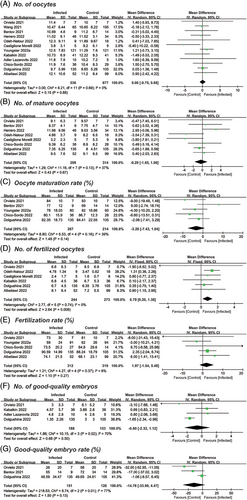
Among the included studies, 12 reported the number of oocytes retrieved, comprising 536 infected versus 677 uninfected cycles.14, 15, 24, 26-29, 31, 32, 34-36 The mean oocyte number was estimated to be 10.67 versus 10.61 (MD = 0.06; 95% CI: −0.75–0.88; p = .88) after meta-analysis (Figure 3A). Six studies (n = 517) provided information on the number of fertilized oocytes,14, 26, 27, 31, 35, 36 and a significant increase was noticed in the infected group (MD = 0.79; 95% CI: 0.20–1.38; p = .008) (Figure 3D). The pooled results of the other laboratory parameters were comparable in the number of mature oocytes (8 studies; MD = −0.29; 95% CI: −1.65–1.06; p = .67),14, 15, 26-29, 31, 36 oocyte maturation rate (five studies; MD = −3.20; 95% CI: −7.43–1.04; p = .14),14, 15, 28, 34, 36 fertilization rate (five studies; MD = 1.97; 95% CI: −1.54–5.48; p = .27),14, 26, 28, 34, 36 number of good-quality embryos (four studies; MD = −0.60; 95% CI: −2.32–1.12; p = .50),14, 24, 35, 36 and good-quality embryo rate (three studies; MD = −14.76; 95% CI: −33.99–4.47; p = .13)14, 15, 36 (Figure 3B,C,E–G). Among the above seven comparisons, the heterogeneity was identified as low in most outcomes, except for the number and rate of good-quality embryos, whose I2 were 70% and 77%, respectively.
3.4 Meta-analysis of pregnancy outcomes
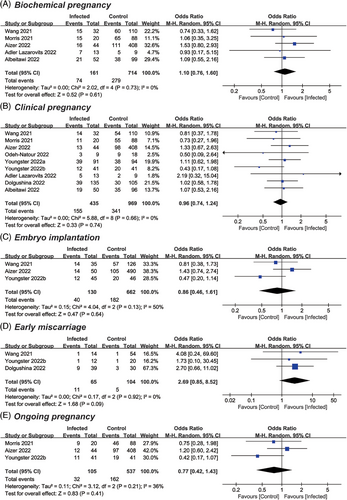
Data on clinical pregnancy rate was provided by nine studies, including a total of 435 previously infected and 969 uninfected cycles.24-26, 30-34, 36 After pooled analysis, the mean OR was calculated to be 0.96 with a 95% CI of 0.74–1.24 (p = .74; I2 = 0) (Figure 4B). There was also no significant impact identified on other pregnancy outcomes. In detail, the ORs were 1.10 for biochemical pregnancy (95% CI: 0.76–1.60; p = .61; I2 = 0),24-26, 30, 32 0.86 for embryo implantation (95% CI: 0.46–1.61; p = .64; I2 = 50%),25, 32, 33 2.69 for early miscarriage (95% CI: 0.85–8.52; p = .09; I2 = 0),32, 33, 36 and 0.77 for ongoing pregnancy (95% CI: 0.42–1.43; p = .41; I2 = 36%)25, 30, 33 (Figure 4A,C–E).
3.5 Subgroup analysis
As shown in Figure S1, the pooled result of oocyte number remained consistently comparable in retrospective (MD = −0.15; 95% CI: −1.42–1.11; p = .81) and prospective (MD = 0.20; 95% CI: −1.06–1.47; p = .75) cohort studies. Moreover, no significant change was observed in subgroups according to sample size ≥ 100 (MD = 0.22; 95% CI: −0.70–1.13; p = .64) and < 100 (MD = −0.51; 95% CI: −2.27–1.25; p = .57). Similarly, the clinical pregnancy rate remained stable in retrospective and prospective design subgroups, with pooled ORs of 0.94 (95% CI: 0.70–1.28; p = .70; I2 = 0) and 1.00 (95% CI: 0.60–1.68; p = .99; I2 = 0), respectively (Figure S2). Also, there was no significant alteration by different sample sizes (≥ 100: OR = 1.03; 95% CI: 0.78–1.36; p = .82; < 100: MD = 0.59; 95% CI: 0.26–1.32; p = 0.20). There was little to no heterogeneity across the meta-analyses (I2 = 0%–21%).
3.6 Sensitivity analysis
The two primary outcomes of retrieved oocyte number and clinical pregnancy rate remained stable when one study was omitted at a time (Figure S3A,B). However, when we removed the paper by Odeh-Natour et al.,31 the correlation between previous COVID-19 infection and the number of fertilized oocytes was rendered insignificant (MD = 0.47; 95% CI: −0.28–1.22) (Figure S3C).
3.7 Publication bias
According to Egger's test, no significant publication bias was found in any of the 15 outcomes analyzed (p = .197–.976), except for the embryo implantation rate (p = .030), as shown in Table S2.
4 DISCUSSION
This systematic review and meta-analysis included 15 studies totalling 1905 cycles to investigate the effect of prior female COVID-19 infection history on ART treatment. Our data suggested that a history of COVID-19 infection in females did not adversely affect subsequent cycle characteristics, laboratory indicators and pregnancy results.
Notably, in our preliminary analysis, the number of fertilized oocytes was increased by 0.79 in the infected group than the control. Nonetheless, when the study by Odeh-Natour et al.31 was removed, this difference was no longer statistically significant, suggesting that data from this single study has a large leverage on the overall result. In the study, 59 women were enrolled over the same period and cycles were grouped based on serum anti-spike and anti-nucleotide antibody levels. However, the age of infected women was younger than that of the control group (31.9 ± 6.4 vs. 35.7 ± 7.0). Consequently, this pooled data should be considered with caution since age may potentially bias the number of fertilized oocytes (median: 5 vs. 3) given its major role in both oocyte quantity and quality.37
Despite the lack of sufficient clinical evidence, SARS-CoV-2 may potentially impact the female reproductive system in multiple biological ways. At present, there is no compelling proof that SARS-CoV-2 could directly attack reproductive organs such as ovary and endometrium,38-41 but viral infection may have an indirect effect on fertility. In a recent study, endometrial gene expression was shown to be altered in 75% of COVID-19 patients, involving pathways related to host immune response, cytokine inflammation, and lipid metabolism.41 Among severe COVID-19 cases, the mean telomere length of granulosa cells was found to be significantly reduced (96.1 ± 21.9 vs. 141.4 ± 67.5; p = .017), which could possibly lead to an unfavourable microenvironment for follicular growth and maturation.28 In addition, Herrero et al.29 demonstrated that previous infection induced the occurrence of anti-SARS-CoV-2 IgG antibody in 91.3% of follicular fluid samples, which may dysregulate ovarian function by altering the expression of steroidogenic enzymes and vascular endothelial growth factor in granulosa cells, impairing migration of endothelial cells, as well as severely damaging DNA stability and integrity in both cells. However, the pooled results in our meta-analysis revealed that prior COVID-19 infection had no appreciable detrimental impact on ART treatment, indicating that the hypothesized pathophysiological mechanisms may not be extrapolated to clinical practice.
Earlier studies suggested that preconception exposure to a serious systemic infection, including sepsis, respiratory failure, and critical care evaluation within 6 months, could cause reproductive sequelae such as increased risk of pregnancy loss.42 The time interval between COVID-19 recovery and oocyte retrieval or ET may also affect ART outcomes, but there are few focused studies on this issue. In the first study conducted by Orvieto et al.,14 the number of top-quality embryos, defined as ≥ 7 equally-sized blastomeres with ≤ 10% fragmentation on day 3, was significantly decreased in the immediate ART cycle following COVID-19 (8–92 days). Thus, they recommended treatment at least 3 months after recovery from infection, as folliculogenesis and spermatogenesis are estimated to be longer than this time span.43, 44 However, this finding was contradicted by another retrospective cohort study showing that fresh ART outcomes were not affected by SARS-CoV-2 infection within 90 days, while a potential long-term adverse effect was observed on oocyte yield after > 180 days.34 In terms of frozen-thawed ET (FET), Youngster et al.33 stratified patients by ≤ 60 days and > 60 days post-COVID-19, and the former group exhibited significantly lower clinical and ongoing pregnancy rates compared with control. Therefore, they recommended that FET should be postponed for at least 60 days following COVID-19 recovery. However, a meta-analysis could not be performed given the different grouping criteria in each study. Since the study number and sample size are limited, further large cohorts are also warranted to clarify the effect of post-COVID-19 interval on ART outcomes.
As far as we know, this is the first meta-analysis on the impact of past COVID-19 infection in females on ART outcomes. There was a low heterogeneity and lack of publication bias in most included studies. Moreover, the primary outcome measures of retrieved oocyte number and rate of clinical pregnancy remained consistent in subgroup and sensitivity analyses, which should strengthen the reliability and robustness of the results.
Several potential limitations still exist in the current study. First, given the lack of adjusted estimates, the pooled analysis was based on crude data. However, most included studies are retrospective cohorts with relatively small sample sizes, which have inherent bias and residual confounding risk. For example, the status of vaccination was not adequately considered,14, 15, 24, 25, 27, 30, 31 while vaccinated women should be deemed immunized as those infected and separated from analysis. In addition, most studies did not take male COVID-19 infection into account, which may lead to decreased blastocyst formation and available blastocyst rates as previously reported.45 The outcome measures were also not uniformly defined across studies, thus affecting the accuracy of synthesized results. Second, no studies have focused on the detailed SARS-CoV-2 variants such as Delta and Omicron, which might pose different effects on ART outcomes given their evolving transmissibility and pathogenicity. Moreover, only one study to date has made comparisons between mild and severe cases of COVID-19 and found a lower oocyte maturation rate only in the latter group.28 In this regard, further studies should differentiate disease severity for clarification. Finally, all studies had a relatively limited follow-up period and no live birth outcomes were reported. Consequently, the results must be construed with caution, and more studies are warranted on the obstetric and neonatal risks of ART women recovering from COVID-19.
For infertile women who recovered from COVID-19, the present finding could provide real-world evidence for their medical consultations on subsequent ART success. Since these women are often confronted with higher levels of anxiety and depression,46 our reassuring data may also help to reduce the psychiatric stress and further protect them from potential negative consequences.47-49 Of note, this study did not preclude the importance of timely COVID-19 vaccination, which has shown its effectiveness in reducing morbidity and mortality as well as safety for assisted reproductive outcomes.50, 51
5 CONCLUSION
To conclude, our systematic review and meta-analysis revealed that prior COVID-19 infection in females had no detrimental effect on ART outcomes, including cycle characteristics, laboratory parameters, as well as pregnancy results. This finding could provide reassuring information for ART women after recovering from COVID-19. More data are needed to investigate the live birth outcome and the optimal time interval from infection to treatment initiation.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (82260315), Natural Science Foundation of Jiangxi Province (20224BAB216025), and Central Funds Guiding the Local Science and Technology Development (20221ZDG020071).
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article and its additional files.




