Inflammation and autonomic balance in cirrhosis: Association between sympathetic nervous system and osteopontin, interleukin-22, interleukin-6 and interleukin-1Ra concentrations according to portal hypertension and disease severity
Abstract
Background
The autonomic nervous system is linked to hyperdynamic circulation in cirrhosis and several studies have highlighted the crucial role that systemic inflammation elicits in altering sympathovagal equilibrium with the consequent reduction in heart rate variability (HRV).
To investigate the correlation between time-domain HRV parameters, serum cytokines concentrations and portal hypertension, we studied a cohort of patients with cirrhosis, accounting for etiology and treatments.
Methods
In this cross-sectional, observational cohort study, 107 outpatients with non-alcoholic cirrhosis were assessed consecutively by abdominal ultrasound and by upper gastrointestinal endoscopy to search for esophagogastric varices. 24-h electrocardiogram Holter monitoring with time-domain HRV measurement (square root of the mean of successive differences of Normal-to-Normal [NN] [RMSSD], standard deviation or the square root of variance [SDNN] and standard deviation of the means of the NN intervals calculated over a 5-min period [SDANN]) was performed and serum concentrations of osteopontin (OPN), interleukin (IL)-22, IL-6, IL-1Ra and IL-17 were obtained in all patients.
Results
IL-6, OPN, IL-22 and IL-1Ra concentrations in cirrhotic patients were associated with disease severity expressed by Child-Pugh and MELD score, to some portal hypertension's indirect signs and some of its complications. A significant increase in systemic concentrations of OPN in patients with hepatocellular carcinoma was encountered. SDANN and SDNN values were indirectly related to serum levels of IL-6, OPN, IL-1Ra and IL-22.
Conclusions
This study underlines the interaction between the alteration of the ANS and the activation of inflammatory pathways that characterize cirrhosis taking into account clinical characteristics and treatments.
1 INTRODUCTION
Esophageal varices (EV) formation and bleeding are the main consequences of portal hypertension and represent an important cause of mortality and morbidity in patients with liver cirrhosis.1 Portal hypertension and hyperdynamic circulation lead to a lowering in mean arterial pressure and activation of neurohormonal mechanisms (the renin-angiotensin system, the sympathetic nervous system [SNS] and vasopressin/ADH) that determine water and NaCl retention. The activation of these three neurohormonal systems is directly proportional to the degree of splanchnic vasodilation and, therefore, to hemodynamic insufficiency and the severity of liver cirrhosis. Heart rate variability (HRV) is a low-cost and noninvasive method that has been validated in patients with ischemic heart disease2-4 and diabetes3 to assess the sympathovagal balance and to explore the activity and functioning of the autonomic nervous system in cirrhosis by analyzing the variability of the so-called Normal-to-Normal (NN) intervals of QRS complexes.5 In the setting of cirrhosis short-term recording and long-term recording HRV parameters have been considered to be indicative of poor prognosis in patients with cirrhosis independently to Meld Score.6, 7 Hepatic fibrosis, which histologically characterizes cirrhosis, is the result of an exalted regenerative response associated with chronic liver damage. Numerous components of the immune system are involved in the genesis of liver fibrosis through the control of myofibroblasts8 and, recently, several studies have evaluated the expression of some inflammatory proteins in patients with cirrhosis, in the attempt to use them as markers of liver damage, the severity of portal hypertension and to predict the onset of other complications. Among these, interleukin-22 (IL-22), IL-6, IL-17 and the receptor antagonist of IL-1 (IL-1Ra) seem to be more associated with portal hypertension and other cirrhosis complications.9-12 Despite the costs and the invasiveness, the measurement of the hepatic venous pressure gradient (HVPG) represents the only truly reliable and accurate tool for the diagnosis and follow-up of portal hypertension. The interest of the research in finding inflammatory biomarkers, potentially used as an indirect measure of HVPG, is based on the fact that portal hypertension is intimately linked to liver damage and fibrosis and these, in turn, are connected to the activation of specific inflammatory pathways.13, 14 Interestingly, a recent review underlined the importance of the link existing between HRV and inflammation in cirrhosis prognosis.15 The authors concluded, that HRV analysis has modified the scenario of prognosis assessment in patients with cirrhosis.
2 AIMS OF THE STUDY
2.1 The main aims of the study were
- – Evaluating any associations between the concentrations of the cytokines examined and time-domain HRV variables in an attempt to investigate the link between sympathovagal balance and inflammation in patients with cirrhosis.
- 1. evaluating the association between the severity of liver cirrhosis, expressed by CHILD PUGH class and MELD score, and osteopontin (OPN), IL-22, IL-6, IL-1Ra and IL-17.
- 2. evaluating the associations between the concentrations of the cytokines examined and all the anamnestic, clinical, endoscopic, ultrasound and blood chemistry variables.
- 3. evaluating the possible association between the severity of portal hypertension, expressed through clinical, endoscopic, ultrasound indirect signs, and the expression of OPN, IL-22, IL-6, IL-1Ra and IL-17 to identify a biomarker potentially able to estimate indirectly the severity of portal hypertension.
- 4. Investigating the potential role of one or more cytokines in predicting the presence of EV as well as their recent bleeding.
3 METHODS
We enrolled all consecutive outpatients affected by liver cirrhosis who were referred to the Digestive Endoscopy Surgery of the Gastroenterology and Hepatology department of the University Hospital “Paolo Giaccone” in Palermo for endoscopic diagnosis or follow-up of portal hypertension from January 2021 to December 2022. In all patients, liver cirrhosis was diagnosed by clinical and ultrasound criteria and, in doubtful cases, by liver biopsy. The study protocol was approved by the “Paolo Giaccone” University Hospital Polyclinic ethics committee (protocol number 052003), and all patients adhered to the study protocol by signing a written informed consent. Patients underwent a complete medical history, physical examination, routine blood tests after 8 h of fasting (glycaemia, creatinine, azotemia, blood count, Aspartase Aminotransferase (AST) and Alanine aminotransferase (ALT), albuminemia, International Normalized Ratio (INR), total bilirubin, Gamma glutamyl transpeptidase (GGT), natremia). Abdominal ultrasound, Esophagogastroduodenoscopy (EGDS) and 24-h electrocardiogram (ECG) Holter monitoring with HRV measurement according to time domain analysis were also performed in all patients. Finally, serum concentrations of OPN, IL-22, IL-6, IL-1Ra and IL-17 were obtained for measurement. The blood samples were obtained in the morning after at least 10 h of fasting, then they were centrifugated and the sera were stored by cryopreservation at −80°. The sample size was estimated to detect a 20% difference in the concentrations of the cytokines and time-domain HRV variables examined, evaluating the association with the different severity parameters of liver cirrhosis. The sample size of 100 patients was calculated to provide 80% power with α = 0.05.
A total of 174 subjects were initially included in the study. Biopsy was performed just in patients (N = 12) in which the diagnosis of cirrhosis was not attributable to clinical evaluation. Afterwards, 67 patients were not included in the final study because of the following exclusion criteria. Patients in whom biopsy or other diagnostic tests have excluded the diagnosis of liver cirrhosis (n = 5), were also excluded from the study. We considered as exclusion criteria the following characteristics: alcohol-related cirrhosis (n = 32) due to the effects of chronic alcohol intake on the ANS, previously known ischemic heart disease (n = 18), supraventricular arrhythmias (especially atrial flutter, persistent or permanent paroxysmal atrial fibrillation) (n = 10), the presence of an endocavity definitive pacemaker (n = 2).
A control group of 50 healthy subjects, matched by gender and average age, was also recruited from blood donors of the U.O. of Transfusion Medicine of the AOUP P. Giaccone of Palermo. Serum cytokine quantification was carried out using the sandwich ELISA technique to measure natural and recombinant human complement factor H. Measurements were carried out at the “Clinical Biochemistry and Clinical Molecular Biology” laboratory of the AOUP P. Giaccone in Palermo in accordance with the specific instructions indicated by the manufacturer of the kits (Bio-Teche, Milan).
For the execution of the 24-h ECG Holter, Sorin Spiderview Digital Holter Recorder devices were used and the analysis of the collected data was carried out through the ELA Medical SyneScope version 3.10 program. The device was placed between 08.30 and 09.30 in the morning and worn by the patient for 24 h. The Holter recorder involved the placement of ten electrodes in the precordial region.
The Holter examination, with HRV measurement according to a time domain analysis, abdominal ultrasound and upper gastrointestinal endoscopy was performed sequentially on different days and in random order within the same month, and 48% of the patients underwent an endoscopy first.
4 DATA ANALYSIS
For the descriptive analysis of the variables under study, frequencies and mean ± standard deviation were used. Differences between groups were assessed using the chi-square test for categorical variables and the t-Student test for continuous variables. Univariate analysis of variance and post-hoc analysis for intra-group comparisons with the Bonferroni Test were performed to assess the difference in cytokine concentrations between the groups. The statistically significant variables in the univariate analysis were identified and used in a multivariate analysis to evaluate the significant association between cytokines and clinical and HRV variables. Receiver operating characteristic curves with calculations of the area under the curve and 95%CI were constructed. Sensitivity and specificity values were calculated to evaluate the ability of cytokines in predicting the presence of EV and their recent bleeding. Data were analyzed by the Epi Info software (version 6.0, Centers for Disease Control and Prevention, Atlanta, GA, USA), and SPSS Software (version 21.0, SPSS Inc, Chicago, IL, USA). All P-values were two-sided, and P-values less than 0.05 were considered statistically significant.
5 RESULTS
According to the abovementioned exclusion and inclusion criteria, a total of 107 patients completed the study. They consisted of 64 men and 43 women aged between 31 and 86 years (mean age 67.3 years). Seventeen patients (15.9%) did not present endoscopic signs of varices, 41 patients (38.3%) had small varices (F1) and 49 patients (47.8%) had large varices (F2 or F3). Data regarding clinical, ultrasound and endoscopic characteristics are summarized in Table 1. There was a male-over-female prevalence (M = 59.8%, F = 40.2%). In about 76.6% of patients, viral infection (HCV and HBV) was considered the etiology of liver cirrhosis. In 15.9% of cases NASH -related cirrhosis was diagnosed and in the remaining 7%, the etiology was considered cryptogenic. Most of the subjects recruited belonged to Child-Pugh Class A (n = 71, 64%), about a quarter of the sample belonged to Child-Pugh Class B (n = 27, 25.2%) and the remaining patients belonged to Child Class C (n = 9; 7.5%).
| Variables | N = 107 | % |
|---|---|---|
| Sex | ||
| Male | 63 | 59.8 |
| Female | 43 | 40.2 |
| Cirrhosis etiopathogenesis | ||
| HCV | 76 | 71 |
| HBV | 6 | 5.6 |
| NASH | 17 | 15.9 |
| Cryptogenic | 2 | 1.9 |
| Other | 6 | 5.6 |
| Diabetes | 29 | 27.1 |
| Hypertension | 43 | 40.2 |
| Child-Pugh Class: | ||
| A | 71 | 66.4 |
| B | 27 | 25.2 |
| C | 8 | 7.5 |
| MELD Score | ||
| 6–10 | 74 | 69.1 |
| 11–16 | 24 | 24.2 |
| 17–20 | 3 | 3.7 |
| >20 | 1 | 0.9 |
| Presence of Ascites | 42 | 39.3 |
| SBP | 5 | 4.6 |
| Portal thrombosis | 11 | 10.3 |
| PSE | 9 | 8.4 |
| HCC | 24 | 22.4 |
| Presence of EV | 90 | 84.1 |
| EV dimension | ||
| No EV | 17 | 15.9 |
| F1 | 41 | 38.3 |
| F2/F3 | 49 | 45.8 |
| Red Weal Mark | 10 | 13.5 |
| EV Recent bleeding | 18 | 16.8 |
| Splenomegaly | 76 | 71 |
- Abbreviations: EV, esophageal varices; HCC, hepatic cell carcinoma; PSE, portosystemic encephalopathy; SBP, spontaneous bacterial peritonitis.
On univariate regression analysis, the mean values of IL-6 were directly related to cryptogenic etiology. IL-6 was also directly related to Child Pugh's class, as well as with the MELD score, APRI score and with the presence of ascites (Table 2). A statistically significant association was also found between IL-6 levels and diagnosis of PSE (Table 3), use of furosemide and use of potassium canreonate (Table S1). Regarding the blood chemistry variables, IL-6 showed a statistically significant inverse association with hemoglobin, natremia, albumin and glomerular filtrate; instead, it was directly related to INR, total bilirubin, AST and creatinine (Table S1). On univariate regression analysis with HRV variables, IL-6 showed a statistically significant inverse association with SDANN 5 min 24 h and SD 24 (Table 4). Upon multivariate analysis, the associations between IL-6 with Child-Pugh class and score, PSE, INR and SDNN24 h were confirmed to be statistically significant (Table 5).
| Variables | IL-6 | OPN | IL-1 Ra | IL-22 | IL-17 | |
|---|---|---|---|---|---|---|
| Child Pugh Class | B | 37.873 | 3.521 | 274.893 | 13.471 | −0.026 |
| P | 0.0001 | 0.0001 | 0.031 | 0.0001 | 0.966 | |
| MELD SCORE | B | 6.359 | 0.387 | 66.295 | 2.461 | −0.021 |
| 0.0001 | 0.008 | 0.002 | 0.0001 | 0.837 | ||
| Cirrhosis etiopathogenesis* | B | 12.054 | 0.311 | 150.146 | 1.992 | 0.390 |
| P | 0.001 | 0.500 | 0.035 | 0.239 | 0.221 | |
| Viral eradication in HCV group | B | −20.178 | −2.971 | −154.181 | −6.922 | −1.406 |
| P | 0.024 | 0.006 | 0.368 | 0.086 | 0.063 | |
| APRI score | B | 10.544 | 0.608 | 156.740 | 6.175 | 0.137 |
| P | 0.0001 | 0.0 53 | 0.001 | 0.0001 | 0.531 |
- APRI, AST to platelet ratio index.
- * cryptogenic form presented higher levels of IL-6 and IL-1Ra compared to the viral forms.
| Variables | IL-6 | OPN | IL-1 Ra | IL-22 | IL-17 | |
|---|---|---|---|---|---|---|
| Presence of Ascites | B | 40.496 | 4.876 | 205.681 | 15.980 | 0.709 |
| P | 0.0001 | 0.0001 | 0.236 | 0.0001 | 0.359 | |
| PSE | B | 79.090 | 4.693 | 793.091 | 28.861 | 1.394 |
| P | 0.0001 | 0.016 | 0.008 | 0.0001 | 0.305 | |
| Portal thrombosis | B | 16.677 | 4.443 | −170.893 | 0.698 | 0.009 |
| P | 0.256 | 0.014 | 0.541 | 0.916 | 0.994 | |
| HCC | B | 6.594 | 4.174 | −24.717 | 4.806 | 0,.05 |
| P | 0.538 | 0.001 | 0.903 | 0.317 | 0.908 | |
| EV dimensions | B | 6.689 | 0.953 | −15.184 | 2.066 | −0.065 |
| P | 0.153 | 0.095 | 0.865 | 0.327 | 0.871 | |
| Portal Vein Diameter | B | 1.411 | 0.449 | 0.500 | −0.003 | −0.222 |
| P | 0.406 | 0.029 | 0.988 | 0.997 | 0.120 |
- Abbreviations: EV, esophageal varices; HCC, hepatic cell carcinoma; PSE, portosystemic encephalopathy.
| Variables | IL-6 | OPN | IL-1 Ra | IL-22 | IL-17 | |
|---|---|---|---|---|---|---|
| 24 h HR | B | 13.309 | 0.080 | 24.872 | 0.417 | 0.042 |
| P | 0.001 | 0.109 | 0.340 | 0.023 | 0.222 | |
| RMSSD 24h | B | 0.0001 | 0.020 | −1.423 | −0.013 | 0.003 |
| P | 0.996 | 0.031 | 0.328 | 0.698 | 0.605 | |
| SDANN 5 min | B | −0.531 | −0.042 | −6.435 | −0.225 | −0.005 |
| P | 0.0001 | 0.014 | 0.016 | 0.0001 | 0.694 | |
| SDNN 24 H | B | − 0.364 | −0.005 | −4.795 | −0.153 | 0.001 |
| P | 0.002 | 0.743 | 0.030 | 0.003 | 0.915 |
- Abbreviations: HR, heart rate; RMSSD, root mean square successive difference of NN intervals; SDANN, standard deviation of the averages of NN intervals; SDNN, standard deviation of the NN intervals.
| Coefficientsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-standardized coefficients | Standardized coefficients | 95.0% Confidence interval for B | ||||||
| Variables | B | Standard error | Beta | t | Sign. | Lower limit | Upper limit | |
| 1 | −43.158 | 64.666 | −0.667 | 0.506 | −171.898 | 85.582 | ||
| Cirrhosis etiopathogenesis | −1.655 | 3.637 | −0.043 | −0.455 | 0.650 | −8.896 | 5.586 | |
| Antiviral Therapy | −30.892 | 11.710 | −0.331 | −2.638 | 0.010 | −54.205 | −7.579 | |
| Virus Eradication | 14.803 | 9.993 | 0.159 | 1.481 | 0.143 | −5.092 | 34.698 | |
| CHILD PUGH CLASS | 40.815 | 16.130 | 0.556 | 2.530 | 0.013 | 8.703 | 72.927 | |
| CHILD PUGH SCORE | −17.149 | 6.158 | −0.689 | −2.785 | 0.007 | −29.408 | −4.889 | |
| MELD SCORE | 0.604 | 1.512 | 0.049 | 0.399 | 0.691 | −2.406 | 3.614 | |
| Presence of Ascites | −17.788 | 13.961 | −0.189 | −1.274 | 0.206 | −45.581 | 10.006 | |
| Severity of Ascites | 24.206 | 7.395 | 0.540 | 3.273 | 0.002 | 9.484 | 38.929 | |
| PSE | 65.544 | 15.311 | 0.398 | 4.281 | 0.0001 | 35.062 | 96.027 | |
| Severity of Portal Gastropathy | 1.847 | 2.972 | 0.049 | 0.621 | 0.536 | −4.071 | 7.764 | |
| FUROSEMIDE | −8.884 | 9.883 | −0.092 | −0.899 | 0.371 | −28.560 | 10.793 | |
| Potassium Canreonato | −2.448 | 10.450 | −0.024 | −0.234 | 0.815 | −23.252 | 18.357 | |
| Hemoglobin | 3.026 | 1.626 | 0.170 | 1.862 | 0.066 | −0.210 | 6.263 | |
| Albumin | −8.829 | 7.715 | −0.127 | −1.144 | 0.256 | −24.189 | 6.532 | |
| INR | 88.572 | 23.669 | 0.368 | 3.742 | 0.0001 | 41.451 | 135.693 | |
| Total Bilirubin | −4.715 | 2.656 | −0.210 | −1.775 | 0.080 | −10.004 | 0.573 | |
| AST | −0.105 | 0.132 | −0.082 | −0.794 | 0.429 | −0.368 | 0.158 | |
| Creatinin | 4.735 | 3.899 | 0.109 | 1.214 | 0.228 | −3.027 | 12.497 | |
| Glomerular filtrate | 0.030 | 0.139 | 0.019 | 0.214 | 0.831 | −0.247 | 0.307 | |
| HR 24 h | 6.941 | 3.079 | 1.608 | 2.254 | 0.027 | 0.810 | 13.071 | |
| Day HR | −2.747 | 1.773 | −0.670 | −1.550 | 0.125 | −6.277 | 0.782 | |
| Night HR | −3.329 | 1.469 | −0.840 | −2.266 | 0.026 | −6.255 | −0.404 | |
| SDANN 5 min 24 h | 0.097 | 0.187 | 0.066 | 0.519 | 0.605 | −0.275 | 0.469 | |
| SDNN 24 h | −0.333 | 0.138 | −0.277 | −2.417 | 0.018 | −0.608 | −0.059 | |
| APRI SCORE | 3.795 | 2.752 | 0.143 | 1.379 | 0.172 | −1.684 | 9.274 | |
- a Dependent variable: IL-6.
- Abbreviations: AST, alanine aminotransferase; HR, heart rate; INR, international normalized ratio; PSE, portosystemic encephalopathy; RMSSD, root mean square successive difference of NN intervals; SDANN, standard deviation of the averages of NN intervals; SDNN, standard deviation of the NN intervals.
OPN values showed a statistically significant direct association with the Child-Pugh class, the MELD score (Table 2) and the presence of ascites. OPN was also significantly and directly related to portal thrombosis, PSE, hepatocellular carcinoma, and portal vein size (Table 3). Furthermore, mean OPN values showed a statistically significant association with the use of furosemide (Table S1). Regarding blood chemistry variables, OPN showed a statistically significant inverse association with hemoglobin, sodium, albumin and glomerular filtrate; instead, it was directly related to white blood cells, INR, total bilirubin, AST, GGT and ALP (Table S1). A trend towards statistical significance was also found in the association between OPN and APRI scores (Table 2). On univariate regression analysis with HRV variables, OPN showed a statistically significant association with 24 h RMSSD; finally, an inverse association with SDANN 5 min 24 h was encountered (Table 4). After multivariate analysis by inserting all the variables found to be statistically significant at the univariate, the associations of OPN with MELD score, portal vein diameter, white blood cells count, glomerular filtrate and 24 h RMSSD were confirmed to be significant (Table 6).
| Coefficientsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Non-standardized coefficients | Standardized coefficients | 95.0% Confidence interval for B | ||||||
| Variables | B | Standard error | Beta | t | Sign. | Lower limit | Upper limit | |
| COSTANT | 26.089 | 22.240 | 1.173 | 0.244 | −18.216 | 70.394 | ||
| 1 | ANTIVIRAL THERAPY | −0.363 | 1.355 | −0.032 | −0.268 | 0.789 | −3.062 | 2.336 |
| VIRUS ERADICATION | −0.532 | 1.316 | −0.047 | −0.404 | 0.687 | −3.154 | 2.090 | |
| CHILD PUGH CLASS | −2.703 | 2.023 | −0.303 | −1.336 | 0.185 | −6.733 | 1.327 | |
| CHILD PUGH SCORE | 0.769 | 0.795 | 0.253 | 0.967 | 0.337 | −0.815 | 2.352 | |
| MELD SCORE | −0.441 | 0.190 | −0.294 | −2.324 | 0.023 | −0.819 | −0.063 | |
| PRESENCE OF ASCITES | −2.357 | 1.964 | −0.206 | −1.200 | 0.234 | −6.269 | 1.555 | |
| SEVERITY OF ASCITES | 1.708 | 1.002 | 0.313 | 1.704 | 0.093 | −0.289 | 3.704 | |
| PORTAL THROMBOSIS | 2.439 | 1.583 | 0.133 | 1.541 | 0.128 | −0.714 | 5.591 | |
| PSE | −1.554 | 1.887 | −0.078 | −0.824 | 0.413 | −5.314 | 2.205 | |
| HCC | 0.424 | 1.123 | 0.032 | 0.378 | 0.707 | −1.813 | 2.662 | |
| EDE | −0.025 | 0.056 | −0.121 | −0.452 | 0.653 | −0.137 | 0.086 | |
| POL | 0.058 | 0.058 | 0.260 | 0.997 | 0.322 | −0.058 | 0.174 | |
| PORTAL VEIN DIAMETER | 0.409 | 0.174 | 0.193 | 20.347 | 0.022 | 0.062 | 0.756 | |
| FUROSEMIDE | 0.080 | 1.046 | 0.007 | 0.076 | 0.939 | −2.003 | 2.163 | |
| HEMOGLOBIN | −0.272 | 0.229 | −0.126 | −10.189 | 0.238 | −0.728 | 0.184 | |
| WHITE CELLS COUNT | 0.0001 | 0.0001 | 0.223 | 2.048 | 0.044 | 0.0001 | 0.001 | |
| SODIUM | −0.212 | 0.146 | −0.144 | −10.447 | 0.152 | −0.503 | 0.080 | |
| ALBUMIN | −0.327 | 1.019 | −0.039 | −0.321 | 0.749 | −2.358 | 1.704 | |
| INR | 4.111 | 3.076 | 0.140 | 10.336 | 0.185 | −2.017 | 10.239 | |
| TOTAL BILIRUBIN | 0.533 | 0.330 | 0.194 | 10.615 | 0.110 | −0.124 | 1.189 | |
| AST | −0.005 | 0.023 | −0.032 | −0.218 | 0.828 | −0.050 | 0.040 | |
| GGT | 0.003 | 0.006 | 0.034 | 0.428 | 0.670 | −0.010 | 0.015 | |
| ALP | −0.003 | 0.011 | −0.029 | −0.269 | 0.789 | −0.025 | 0.019 | |
| GLOMERULAR FILTRATE | −0.056 | 0.017 | −0.301 | −3.354 | 0.001 | −0.089 | −0.023 | |
| RMSSD 24 H | 0.022 | 0.008 | 0.228 | 2.602 | 0.011 | 0.005 | 0.039 | |
| SDANN 5 MIN 24H | 0.001 | 0.015 | 0.007 | 0.085 | 0.933 | −0.028 | 0.031 | |
| APRI SCORE | 0.938 | 0.504 | 0.290 | 1.863 | 0.066 | −0.065 | 1.942 | |
- a Dependent variable: OPN.
- ALP, alkaline phosphatase; AST, alanine aminotransferase; EDE, varices endoscopic dimension estimation; GGT, gamma glutamyl transpeptidase; HCC, hepatocellular carcinoma; HR, heart rate; INR, international normalized ratio; POL: percentage of occupied lumen; PSE, portosystemic encephalopathy; RMSSD, root mean square successive difference of NN intervals; SDANN, standard deviation of the averages of NN intervals.
On univariate regression analysis, mean IL-1Ra values showed a statistically significant association with the etiology of cirrhosis, in particular, the cryptogenic form presented higher IL-1Ra values than the viral forms. IL-1Ra showed also a statistically significant association with Child Pugh's class, MELD score and APRI score (Table 2). On univariate regression analysis of HRV variables, IL-1Ra showed a statistically significant association with HR 24 h and an inverse association with SDANN 5 min 24 h and SD 24 (Table 4). After multivariate analysis by inserting all the variables found to be statistically significant at the univariate, the association of IL-1Ra with the diagnosis of PSE was confirmed to be significant (Table 7).
| Coefficientsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables |
Non-standardized coefficients |
Standardized coefficients | t | Sign. | 95.0% Confidence interval for B | |||
| B | Standard error | Beta | Lower limit | Upper limit | ||||
| 1 | (Costante) | 46.137 | 1125.286 | .041 | .967 | −2188.778 | 2281.052 | |
| ETIOLOGY OF CIRRHOSIS | 62.067 | 66.576 | .090 | .932 | .354 | −70.158 | 194.293 | |
| CHILD PUGH CLASS | 489.589 | 372.013 | .373 | 1.316 | .191 | −249.262 | 1228.439 | |
| CHILD PUGH SCORE | −245.439 | 137.874 | −.551 | −1.780 | .078 | −519.268 | 28.391 | |
| MELD SCORE | 28.879 | 33.636 | .131 | .859 | .393 | −37.926 | 95.684 | |
| PSE | 702.888 | 336.688 | .239 | 2.088 | .040 | 34.196 | 1371.580 | |
| INR | 452.728 | 505.512 | .105 | .896 | .373 | −551.262 | 1456.717 | |
| HR 24h | 41.197 | 69.819 | .534 | .590 | .557 | −97.469 | 179.863 | |
| Day HR | −22.625 | 39.398 | −.308 | −.574 | .567 | −100.874 | 55.623 | |
| Night HR | −3.523 | 33.217 | −.050 | −.106 | .916 | −69.495 | 62.448 | |
| SDANN 5 min 24h | .054 | 4.360 | .002 | .012 | .990 | −8.605 | 8.714 | |
| SDNN 24 h | −.709 | 3.095 | −.033 | −.229 | .819 | −6.855 | 5.438 | |
| APRI | 93.725 | 48.509 | .197 | 1.932 | .056 | −2.619 | 190.069 | |
- a Dependent variable: IL1RA.
- Abbreviations: HR, heart rate; INR, international normalized ratio; PSE, portosystemic encephalopathy; SDANN, standard deviation of the averages of NN intervals; SDNN, standard deviation of the NN intervals.
On univariate regression analysis, mean IL-22 values showed a statistically significant association with Child Pugh's class, MELD score, APRI score (Table 2) and with the presence of ascites. A statistically significant association was also found between IL-22 levels with the diagnosis of PSE (Table 3), use of furosemide and potassium canreonate (Table S1). Regarding the blood chemistry variables, IL-22 showed a statistically significant inverse association with hemoglobin, sodium, albumin and glomerular filtrate; IL-22 was instead directly related to white blood cells count, INR, total bilirubin, AST, ALT, GGT and ALP (Table S1). On univariate regression analysis of HRV variables, IL-22 showed a statistically significant association with HR 24 h and an inverse association with SDANN 5 min 24 h and SD 24 (Table 4). After multivariate analysis by inserting all the variables found to be statistically significant at the univariate, the associations of IL-22 with PES, furosemide, potassium canreonate, ALP, APRI score and SD 24 h were confirmed to be significant (Table 8).
| Coefficientsa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variables |
Non-standardized coefficients |
Standardized coefficients | t | Sign. | 95.0% Confidence interval for B | |||
| B | Standard error | Beta | Lower limit | Upper limit | ||||
| 1 | (Costante) | 2076.725 | 4709.022 | .441 | .660 | −7298.218 | 11451.669 | |
| diabete | −30.520 | 200.563 | −.017 | −.152 | .879 | −429.810 | 368.770 | |
| CHILD PUGH CLASS | 453.106 | 399.682 | .345 | 1.134 | .260 | −342.600 | 1248.812 | |
| CHILD PUGH SCORE | −254.214 | 154.895 | −.571 | −1.641 | .105 | −562.587 | 54.159 | |
| MELD SCORE | 17.896 | 40.706 | .081 | .440 | .661 | −63.144 | 98.936 | |
| ASCITIS PRESENCE | 82.429 | 365.877 | .049 | .225 | .822 | −645.976 | 810.834 | |
| PSE | 754.773 | 360.649 | .257 | 2.093 | .040 | 36.775 | 1472.771 | |
| FUROSEMIDE | 542.620 | 246.326 | .315 | 2.203 | .031 | 52.223 | 1033.017 | |
| POTASSIUM CANRENONATE | −603.983 | 258.379 | −.331 | −2.338 | .022 | −1118.377 | −89.589 | |
| HB | −8.172 | 42.392 | −.026 | −.193 | .848 | −92.567 | 76.223 | |
| WBC | .028 | .038 | .088 | .741 | .461 | −.047 | .103 | |
| SODIUM | −16.086 | 28.654 | −.075 | −.561 | .576 | −73.132 | 40.960 | |
| ALBUMIN | 139.585 | 193.783 | .112 | .720 | .473 | −246.207 | 525.377 | |
| INR | 600.108 | 612.736 | .140 | .979 | .330 | −619.755 | 1819.972 | |
| BILIRUBIN TOT | 45.971 | 69.809 | .114 | .659 | .512 | −93.009 | 184.951 | |
| AST | −1.848 | 4.694 | −.081 | −.394 | .695 | −11.193 | 7.496 | |
| ALT | −.672 | 4.015 | −.028 | −.167 | .867 | −8.666 | 7.321 | |
| GGT | .865 | 1.280 | .074 | .676 | .501 | −1.682 | 3.413 | |
| ALP | −4.366 | 2.103 | −.296 | −2.076 | .041 | −8.552 | −.179 | |
| eGFR | −.444 | 3.305 | −.016 | −.134 | .893 | −7.024 | 6.135 | |
| HR 24 h | 52.627 | 76.439 | .682 | .688 | .493 | −99.551 | 204.806 | |
| Day HR | −31.899 | 42.845 | −.435 | −.745 | .459 | −117.196 | 53.398 | |
| Night HR | −6.493 | 36.431 | −.092 | −.178 | .859 | −79.021 | 66.034 | |
| SDANN 5 min 24 h | −1.296 | 4.922 | −.049 | −.263 | .793 | −11.096 | 8.504 | |
| SDNN 24 h | .486 | 3.641 | .023 | .134 | .894 | −6.763 | 7.735 | |
| APRI | 147.037 | 68.990 | .310 | 2.131 | .036 | 9.689 | 284.384 | |
- a Dependent variable_IL-22.
- ALP, alkaline phosphatase; AST, alanine aminotransferase; GGT, gamma glutamyl transpeptidase; HB, hemoglobin; HR, heart rate; INR, international normalized ratio; PSE, portosystemic encephalopathy; SDANN, standard deviation of the averages of NN intervals; SDNN, standard deviation of the NN intervals; WBC, white blood cells.
On univariate regression analysis, the mean IL-17 values did not show statistically significant associations with clinical, blood chemistry and HRV variables (Tables 2–5). Therefore, no multivariate analysis was performed for IL-17. Finally, receiver operating characteristic (ROC) curve of the predictive rate of cytokines in the presence of esophageal varices and recent variceal bleeding are shown in Figure 1.
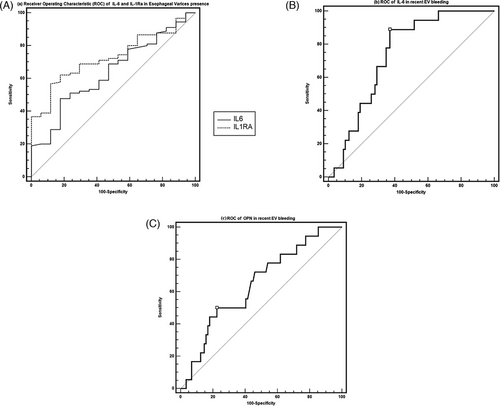
6 DISCUSSION
In this cross-sectional, observational cohort study we evaluated the concentrations of some important pleiotropic and proinflammatory cytokines in 107 patients with non-alcoholic liver cirrhosis of mixed etiology. A 24h- Holter-ECG was recorded in all patients for the determination of time-domain analysis HRV parameters in order to indirectly assess the sympathovagal balance. In this study, we decided to use some specific exclusion criteria to obtain a homogeneous sample and exclude patients with predisposing conditions for severe autonomic neuropathy (e.g. history of alcohol abuse) that could therefore affect the 24-h HRV parameters or conditions that could alter the inflammatory and cytokine response (e.g. sepsis, pancreatitis, severe obesity and other pro-inflammatory conditions). The present study showed that IL-6, OPN, IL-22 and IL1Ra concentrations in cirrhotic patients are largely associated with disease severity expressed by Child-Pugh and MELD score, portal hypertension and some of its complications, as well as sympathovagal alteration expressed by the 24-h HRV parameters.
Concentrations of IL-6 and IL-1 Ra demonstrated a robust association with the cryptogenic form of liver cirrhosis compared to viral ones. In previous studies, IL-6, which represents an over-expressed cytokine in all forms of cirrhosis, has been found to be more associated with alcoholic than viral and cryptogenic forms.16, 17 Our data also includes IL-1Ra together with IL-6, and, although dampened by the poor representation of cryptogenic forms in our sample, may represent an important starting point for the study of the inflammatory processes underlying the forms of cirrhosis of unknown etiology. In fact, both cytokines are linked to the innate immune response due to dysfunction of the intestinal mucosal barrier.18
In our study, we found further confirmations regarding the complex cytokine pattern that characterizes patients with chronic liver disease. In fact, we encountered higher concentrations of IL-6, IL-22, IL-17 and OPN in the group of cirrhotic patients compared to healthy subjects (Table S2). Furthermore, in accordance with literature data,16 the concentrations of IL-6, OPN and IL-1Ra showed a robust association with the severity of liver disease expressed both by Child-Pugh score and by Meld Score.19 This data is also corroborated by the significant association found in subsequent T-student and Bonferroni tests which showed a progressive increase in serum concentrations of IL-6, IL-22, OPN and IL-17 in the three groups of patients divided according to Child-Pugh class (Table S3). The cytokine “milieu” of the cirrhotic patient is, in fact, intimately linked to the anatomopathological alterations and therefore to the expression of the disease at a clinical and molecular level.20 The Child-Pugh and MELD scores, which will reflect the progression of the disease, correlated with the inflammatory substrate underlying these alterations expressed by the complex cytokine pattern. Another interesting association was found in the univariate analysis between the aforementioned cytokines and the APRI score. In a meta-analysis of 40 studies,21 the researchers concluded that an APRI score greater than 1.0 had a sensitivity of 76% and a specificity of 72% for predicting cirrhosis.
APRI alone is probably not sensitive enough to exclude significant diseases.22 Our data, strengthened by the well-known role that these cytokines play in the process of fibrosis and disruption of liver architecture, contributes to making even more suggestive the potential use of IL-6, OPN, IL-22 and IL-1Ra as markers capable of predicting the presence of cirrhosis and liver fibrosis.
6.1 Portal hypertension and complications
Several studies have tried to validate the role of some cytokines, especially IL-6 and IL-1Ra, as indirect measures of the degree of portal hypertension and HVPG,14 also focusing on the association of their serum concentrations and the development of the main complications of portal hypertension.23, 24 In our study we found a strong association between the serum levels of IL-6, OPN and IL-22 and the presence of ascites. Furthermore, the association with IL-6 remained statistically significant in the multivariate analysis after correction for all the significant variables found in the univariate analysis. This data, in agreement with some evidence present in the literature16, 25 can be explained by the elevated concentrations of these cytokines, especially of IL-6, reconverted in the ascitic fluid due to a likely augmented production in the peritoneal cavity.26 In our study we analyzed in particular the expression of the abovementioned cytokines in decompensated cirrhotic patients. We divided our sample into two groups based on the presence or absence of ascites on ultrasound of the abdomen and we evaluated the differences between the two groups in terms of serum cytokine concentrations. In line with the data previously described, we found significantly higher serum levels of IL-6, OPN and IL-22 in decompensated patients than in those without ascites. These data confirm once again the association between these cytokines and the natural history of portal hypertension.20 Some studies have analyzed the link between IL-6 and some conditions secondary to portal hypertension such as PSE24, 26 and portal thrombosis,23 showing a positive association, respectively, with plasma ammonia and clinical severity of PSE and a significant association with coagulation alterations and incidence of portal thrombosis. In our study IL-6 together with OPN, IL-1Ra and IL-22 were shown to be significantly associated with the diagnosis of PSE. Furthermore, the association of IL-6, IL-22 and IL-1Ra with PSE remained statistically significant in the multivariate analysis after correction for all the significant variables found in the univariate analysis.
6.2 Autonomous nervous system and cytokine pattern
One of the main features that provide originality to our study is having investigated in our patients the link between sympathovagal balance and inflammation. The upregulation of the SNS, in the cirrhotic patient, influences the cardiac output, representing an adaptive compensation system that aims to reduce the effective circulating volume. As already highlighted in other studies, the progression of liver pathology and the increase in portal pressure is accompanied by greater activation of the SNS and an increase in hyperdynamic circulation.27, 28 Data regarding the reduction of SDANN and SDNN values, which better express the whole sympathovagal balance, associated with an increase in serum levels of IL-6, OPN, IL-1Ra and IL-22 demonstrate the complexity of the interaction of cytokine pathways and autonomic nervous system. These data highlight the possible point of contact between these two systems, immunomolecular and nervous, just mentioned or only briefly described in some studies but never placed in a prominent position. Epidemiological studies have shown a significant correlation between circulating levels of IL-6 and indices of reduced heart rate variability in various clinical conditions.29 Although these reports suggest a role of IL-6 in reducing heart rate variability during inflammation, evidence of a cause-and-effect relationship is not easy to find in the literature. The “anti-inflammatory reflex” was recently described in a study by Tracey and colleagues30 and refers to the role that the autonomic nervous system plays in regulating host defense and physiological responses to pathogens. The brain appears to modulate systemic inflammatory responses to pathogens by activating vagal efferent fibers. Acetylcholine, the main vagal neurotransmitter, significantly attenuated the release of pro-inflammatory cytokines (e.g. IL-6) through the activation of α7-nicotinic receptors on macrophages.30 In other words, in patients with compensated “anti-inflammatory reflex”, increased vagal activity (high heart rate variability) could suppress IL-6 production (low circulating IL-6). Similarly, patients with impaired “anti-inflammatory reflex” would exhibit inappropriate vagal activity (reduced heart rate variability) and high circulating IL-6 levels associated with a poor prognosis.30 A similar phenomenon has been reported in papillary muscles isolated from cirrhotic rats.31 Jaue et al. reported significantly less inhibition of incremental concentrations of a cholinergic agonist in cirrhotic heart muscles than controls. Cirrhosis, being linked to high circulatory levels of IL-6, is associated with both loss of heart rate variability and reduced response to cholinergic stimulation.32, 33 The authors concluded that cardiac muscarinic receptor density and binding affinity were similar in cirrhotic rat hearts compared to controls and suggested that changes in post-receptor factors could explain the hyporeactivity to cholinergic stimuli. Interestingly, IL-6 has been recognized to be significantly inversely related to decreased HRV variables. In this regard, Mani et al.32 found that augmented IL-6 levels in patients’ plasma were inversely proportional to total HRV and long-term HRV indices. Furthermore, another recent study33 showed that intraperitoneal administration of IL-6 was associated with a significant reduction of SDNN.
Several studies strengthened the concept that systemic inflammation, especially though IL-6, represents an important supportive factor for HRV reduction,32-34 however, the exact role of IL-6 in this mechanism has not been fully elucidated and requires further investigation.
Moreover, decreased HRV in patients with cirrhosis has been related to the increase of cytokine levels,35 mostly IL-629, 36, 37 underlining the central role of systemic inflammation in cirrhosis and HRV reduction.
Furthermore, in an elegant study, Mani et al.32 demonstrated that plasma levels of IL-6 were significantly related with HRV parameters and neuropsychiatric performance in patients with cirrhosis and PSE. The changes observed in HRV and in neuropsychiatric status in patients with cirrhosis suggested a common pathogenic mechanism mediated by inflammatory cytokines.
IL-6 was not the only cytokine studied in this context. In fact, the association between OPN and autonomic functions has also been studied in some diseases such as diabetes and some orthopedic conditions.38, 39 In these studies, OPN was associated with reduced parasympathetic function, especially in young patients with type 2 diabetes mellitus. Although it was not clear what role OPN played towards ANS if protective, harmful or merely bystander. It is possible that, as a mediator of inflammation, the chronic release of OPN could be toxic, potentially leading to neuronal dysfunction and degeneration.40 The cross-sectional nature of former studies, however, did not allow us to determine the role of the OPN and its association with cardiovascular autonomic function. In our study, OPN is not only associated with a reduction in the indices expressing sympathetic activity in the sympathovagal balance in toto (SDANN 5 min and SDNN 24) but is also associated with an increase in RMSSD, which is one of the main HRV parameters that best expresses the parasympathetic activity. In light of these results, we can hypothesize that OPN is therefore associated with an alteration of the sympathovagal balance probably linked to the upregulation of the parasympathetic component. This unpublished finding in the literature however seems to be in line with data previously reported. OPN once again seems to be a candidate as a reliable marker of disease severity and alterations that accompany the evolution of cirrhosis and portal hypertension, such as the alteration of the autonomic balance. In this sense, it is not yet clear whether the relationship between OPN and this sympathetic alteration is harmful or protective but the association found in our study with the upregulation of the parasympathetic nervous system perhaps suggests a more protective role, since some recent evidence supports the effect of parasympathetic activity on the outcome of cirrhotic patients.41 Our study demonstrated a link between autonomic activity and immunomodulation in a large and homogeneous cohort of cirrhotic patients. Moreover, the direct association we found between serum levels of IL-6, IL1-Ra and IL-22 with the average heart rate recorded on the ECG-Holter is worth of interest. Since the increase in heart rate is one of the fundamental elements of the hyperdynamic circulation of the cirrhotic patient, its association with the aforementioned cytokines allows us to imagine once again the close link between inflammatory pathways, hyperdynamic circulation, sympathovagal balance and portal hypertension in patients with cirrhosis.
Finally, we believe that clarifying the link between the inflammation, which underlies the histopathological damage, fibrogenesis and the distortion of the liver architecture, and the sympathetic nervous system which represents an important mechanism of compensation and hemodynamic readjustment as well as a therapeutic target in the patients with cirrhosis and portal hypertension, could be one of the possible keys for identifying future therapies and the correct target of patients to treat.
7 LIMITATIONS OF THE STUDY
Our study presents some relevant limitations. First, the cross-sectional design of the study conditions in some way the interpretations of results. Since correlation does not imply causation, we cannot state the nature of the link found in our study between inflammatory molecules and the expression of sympathovagal activity. Therefore, contemplating the cirrhotic patient a causal relationship between cytokine stimulation mediated by sympathetic activation and, thus, inhibition of the recently described so-called “anti-inflammatory reflex” remains only one of the hypotheses. Secondly, portal hypertension in our patient cohort was not evaluated by precise assessment of pressures using HVPG as the gold standard for correct diagnosis. In fact, in our study, we just considered all the indirect, clinical, endoscopic, ultrasound and blood chemistry signs that characterized portal hypertension and all the secondary complications of these conditions. Almost each of these signs and complications was associated with particular cytokine patterns reflecting the close link between inflammation and clinical expression of portal hypertension and the potential of the inflammatory molecules examined as indirect biomarkers of disease severity.
8 CONCLUSIONS
This study confirms and validates some data already present in the literature concerning the high expression of IL-6, OPN and IL-22 in subjects with liver cirrhosis compared to healthy subjects and the association between these cytokines and the severity of liver cirrhosis expressed by Child Pugh's class and Meld score, the association between OPN and hepatocellular carcinoma and its potential role as a marker of carcinogenesis, as well as the association of IL-6, OPN, IL-1Ra and IL-22 with indirect signs of portal hypertension (clinical, blood chemistry, ultrasound) and its complications (portal thrombosis, PSE, ascites). Furthermore, this study lays the foundations for new researches that intend to investigate the ability of some cytokines such as IL-6, IL1-Ra and OPN in predicting the presence of EV and the ability of these cytokines to identify a population at high risk of relapse of variceal bleeding. Finally, our study underlines the association between the alteration of the ANS and the activation of inflammatory pathways that characterize portal hypertension in chronic liver. Deepening the knowledge of the interaction between ANS and inflammatory regulation could provide insights into the possible impairment of the so called antinflammatory reflex and its role in natural history of cirrhosis and portal hypertension progression. Further studies are needed to investigate this aspect.
CONFLICT OF INTEREST STATEMENT
Vincenza Calvaruso consults and is on the speakers’ bureau for Advanz. She consults for Ipsen and received grants from Gilead. The remaining authors declare no conflict of interest.
FUNDING INFORMATION
None




