Structurally Diverse Limonoids from Trichilia connaroides and Their Antitumor Activities
Ying Yan
State Key Laboratory of Functions and Applications of Medicinal Plants & College of Pharmacy, Guizhou Provincial Engineering Technology Research Center for Chemical Drug R&D, Guizhou Medical University, Guiyang, Guizhou, 550014 China
School of Medicine and Health Management, Guizhou Medical University, Guiyang, Guizhou, 550025 China
These authors contributed equally.
Search for more papers by this authorDan Wang
State Key Laboratory of Functions and Applications of Medicinal Plants & College of Pharmacy, Guizhou Provincial Engineering Technology Research Center for Chemical Drug R&D, Guizhou Medical University, Guiyang, Guizhou, 550014 China
These authors contributed equally.
Search for more papers by this authorFang-Jiao Zhou
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
These authors contributed equally.
Search for more papers by this authorYu-Han Zhao
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorXu-Jie Qin
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorYu Zhang
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorXiao Ding
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorCorresponding Author
Xiao-Jiang Hao
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
E-mail: [email protected]Search for more papers by this authorYing Yan
State Key Laboratory of Functions and Applications of Medicinal Plants & College of Pharmacy, Guizhou Provincial Engineering Technology Research Center for Chemical Drug R&D, Guizhou Medical University, Guiyang, Guizhou, 550014 China
School of Medicine and Health Management, Guizhou Medical University, Guiyang, Guizhou, 550025 China
These authors contributed equally.
Search for more papers by this authorDan Wang
State Key Laboratory of Functions and Applications of Medicinal Plants & College of Pharmacy, Guizhou Provincial Engineering Technology Research Center for Chemical Drug R&D, Guizhou Medical University, Guiyang, Guizhou, 550014 China
These authors contributed equally.
Search for more papers by this authorFang-Jiao Zhou
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
These authors contributed equally.
Search for more papers by this authorYu-Han Zhao
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorXu-Jie Qin
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorYu Zhang
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorXiao Ding
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
Search for more papers by this authorCorresponding Author
Xiao-Jiang Hao
Key Laboratory of Phytochemistry and Natural Medicines, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, Yunnan, 650201 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
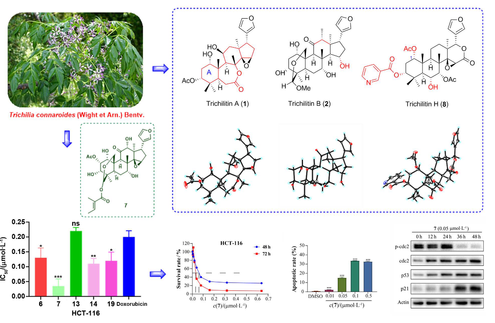
Twelve new limonoids (1—12), named trichilitins A—L, were isolated from the leaves and twigs of Trichilia connaroides, together with ten known compounds (13—22). The structures were elucidated by extensive spectroscopic investigations, X-ray diffraction analyses, and ECD calculations. Compound 1, which belongs to a unique class of ring B-seco limonoid, has been identified as 6/7/6/5 tetracyclic due to a key Baeyer-Villiger oxidation. Compounds 2—7 were identified as ring intact limonoids, while compounds 8—10 were established as ring D-seco ones, and 11 and 12 were determined to be rearranged ones. All of the compounds were tested for cytotoxicity against three human tumor cell lines (HCT-116, NCl-H1975, and SH-SY5Y). Compounds 6, 7, 13, 14, and 19 exhibited significant cytotoxic effects, especially 7 exhibited significant cytotoxic effects against HCT-116 with an IC50 value of 0.035 μmol·L–1 and was more active than the positive control, doxorubicin with an IC50 value of 0.20 μmol·L–1. Compound 7 effectively induced apoptosis of HCT-116, which was associated with S-phase cell cycle arrest. Furthermore, the Western blot analysis showed that compound 7 could induce cell cycle arrest by promoting the expression levels of p53 and p21.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400923-sup-0001-supinfo.pdfPDF document, 17.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Solipeta, D. R.; Bandi, S.; Vemulapalli, S. P. B.; Pallavi, P. M. C.; Vemireddy, S.; Balasubramania, S.; Mahabalarao, S. K. H.; Katragadda, S. B. Secophragmalin-type limonoids from Trichilia connaroides: isolation, semisynthesis, and their cytotoxic activities. J. Nat. Prod. 2019, 82, 2731–2743.
- 2 Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D. M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953.
- 3 Choudhari, A. S.; Mandave, P. C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol. 2019, 10, 1614.
- 4 Newman, D. J.; Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335.
- 5 Cragg, G. M.; Grothaus, P. G.; Newman, D. J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043.
- 6 Luo, J.; Sun, Y. P.; Li, Q. R.; Kong, L. Y. Research progress of meliaceous limonoids from 2011 to 2021. Nat. Prod. Rep. 2022, 39, 1325–1365.
- 7 Braga, T. M.; Rocha, L.; Chung, T. Y.; Oliveira, R. F.; Pinho, C.; Oliveira, A. I.; Morgado, J.; Cruz, A. Biological activities of gedunin-A limonoid from the Meliaceae family. Molecules 2020, 25, 493–501.
- 8 Fernandes, S. R.; Barreiros, L.; Oliveira, R. F.; Cruz, A.; Prudencio, C.; Oliveira, A. I.; Pinho, C.; Santos, N.; Morgado, J. Chemistry, bioactivities, extraction and analysis of azadirachtin: State-of-the-art. Fitoterapia 2019, 134, 141–150.
- 9 Sandhir, R.; Khurana, M.; Singhal, N. K. Potential benefits of phytochemicals from Azadirachta indica against neurological disorders. Neurochem. Int. 2021, 146, 105023–105036.
- 10 Nagini, S.; Nivetha, R.; Palrasu, M.; Mishra, R. Nimbolide, a neem limonoid, is a promising candidate for the anticancer drug arsenal. J. Med. Chem. 2021, 64, 3560–3577.
- 11 Tan, Q. G.; Luo, X. D. Meliaceous limonoids: chemistry and biological activities. Chem. Rev. 2011, 111, 7437–522.
- 12 Jiangsu New Medical College. Dictionary of Chinese Crude Drugs, Shanghai Scientific Technologic Press, Shanghai, 1977, pp. 1925–1926.
- 13 An, F. L.; Luo, J.; Li, R. J.; Luo, J. G.; Wang, X. B.; Yang, M. H.; Yang, L.; Yao, H. Q.; Sun, H. B.; Chen, Y. J.; Kong, L. Y. Spirotrichilins A and B: two rearranged spirocyclic limonoids from Trichilia connaroides. Org. Lett. 2016, 18, 1924–1927.
- 14 Wang, G. C.; Fan, Y. Y.; Shyaula, S. L.; Yue, J. M. Triconoids A–D, four limonoids possess two rearranged carbon skeletons from Trichilia connaroides. Org. Lett. 2017, 19, 2182–2185.
- 15
Chen, A. H.; Wen, Q.; Ma, Y. L.; Jiang, Z. H.; Liu, Q. L.; Tang, J. Y.; Xu, W.; Liu, Y. P.; Fu, Y. H. Bioactive mexicanolide-type limonoids from the fruits of Trichilia connaroides. Phytochem. Lett. 2017, 20, 17–21.
10.1016/j.phytol.2017.03.008 Google Scholar
- 16 Liu, C. P.; Xu, J. B.; Han, Y. S.; Wainberg, M. A.; Yue, J. M. Trichiconins A–C, limonoids with new carbon skeletons from Trichilia connaroides. Org. Lett. 2014, 16, 5478–5481.
- 17
Kraus, W.; Grimminger, W. Toonafolin, einneues tetranortriterpenoid-B-lactonaus Toona ciliata M. J. Roem. var. australis (Meliaceae). Liebigs Ann. Chem. 1981, 1, 1838–1843.
10.1002/jlac.198119811011 Google Scholar
- 18 Su, Z. S.; Yang, S. P.; Zhang, S.; Dong, L.; Yue, J. M. Meliarachins A–K: eleven limonoids from the twigs and leaves of Melia azedarach. Helv. Chim. Acta 2011, 94, 1515–1526.
- 19 Carpinella, M. C.; Defago, M. T.; Valladares, G.; Palacios, S. M. Antifeedant and insecticide properties of a limonoid from Melia azedarach (Meliaceae) with potential use for pest management. J. Agric. Food Chem. 2003, 51, 369−374.
- 20
Nakatan, M.; Huang, R. C.; Okamura, H.; Naoki, H.; Iwagawa, T. Limonoid antifeedants from chinese Melia azedarach. Phytochemistry 1994, 36, 39–41.
10.1016/S0031-9422(00)97008-0 Google Scholar
- 21 Liu, S. B.; Mei, W. L.; Chen, H. Q.; Wang, J.; Wang, Z. N.; Dai, H. F. Limonoids from the roots of Trichilia sinensis and their cytotoxicities. Arch. Pharm. Res. 2018, 41, 1170–1177.
- 22 Yuan, C. M.; Zhang, Y.; Tang, G. H.; Li, Y.; He, H. P.; Li, S. F.; Hou, L.; Li, X. Y.; Di, Y.T.; Li, S. L.; Hua, H. M.; Hao, X. J. Cytotoxic limonoids from Melia azedarach. Planta Med. 2013, 79, 163–168.
- 23 Sun, Y. J.; Cui, L. T.; Sun, Y. P.; Li, Q. R.; Li, Y.Y.; Wang, Z. F.; Xu, W. J.; Kong, L. Y.; Luo, J. A/D-rings-limonoids from the fruits of and their bioactivities. Phytochemistry 2022, 195, 113049–113058.
- 24 Ning, J.; Di, Y. T.; Fang, X.; He, H. P.; Wang, Y. Y.; Li, Y.; Li, S. L.; Hao, X. J. Limonoids from the leaves of Cipadessa baccifera. J. Nat. Prod. 2010, 73, 1327–1331.
- 25 Xu, J. B.; Lin, Y.; Dong, S. H.; Wang, F.; Yue, J. M. Trichinenlides A−T, mexicanolide type limonoids from Trichilia sinensis. J. Nat. Prod. 2013, 76, 1872–1880.
- 26
Woong, J.; Choi, S. U.; Lee, C. O. Cytotoxic limonoids from Melza Azedach var. Japonica. Phytochemistry 1994, 36, 1493–1496.
10.1016/S0031-9422(00)89749-6 Google Scholar
- 27 Carpinella, M. C.; Defago, M. T.; Valladares, G.; Palacios, S. M. Antifeed ant and insecticide properties of a limonoid from Melia azedarach (Meliaceae) with potential use for pest management. J. Agric. Food Chem. 2003, 51, 369–374.
- 28 Nakatani, M. Limonoids from Melia toosendan (Meliaceae) and their antifeedant activity. Heterocycles 1999, 50, 595–609.
- 29 Zhou, H. L.; Hamazaki, A.; Fontna, J. D.; Takahashi, H.; Wandscheer, C. B.; Fukuyama, S. Y. Cytotoxic limonoids from brazilian Melia azedarach. Chem. Pharm. Bull. 2005, 53, 1362–1365.
- 30 Su, Z. S.; Yang, S. P.; Zhang, S.; Dong, L.; Yue, J. M. Meliarachins A–K: eleven limonoids from the twigs and leaves of Melia azedarach. Helv. Chim. Acta 2011, 94, 1515–1526.
- 31 An, F. L.; Yin, Y.; Luo, J.; Kong, L. Y. Highly oxygenous trichilin-type limonoids from Trichilia sinensis. Chin. J. Nat. Med. 2019, 17, 912–917.
- 32 Zhu, G. Y.; Bai, L. P.; Liu, L.; Jiang, Z. H. Limonoids from the fruits of Melia toosendan and their NF-κB modulating activities. Phytochemistry 2014, 107, 175–181.
- 33 Wang, X. N.; Fan, C. Q.; Yin, S.; Gan, L. S.; Yue, J. M. Structural elucidation of limonoids and steroids from Trichilia connaroides. Phytochemistry 2008, 69, 1319–1327.
- 34 Najmuldeen, I. A.; Hadi, A. H. A.; Awang, K.; Mohamad, K.; Ketuly, K. A.; Mukhtar, M. R.; Chong, S. L.; Chan, G.; Nafiah, M. A.; Weng, N. S.; Shirota, O.; Hosoya, T.; Nugroho, A. E.; Morita, H. Chisomicines A−C, Limonoids from Chisocheton Ceramicus. J. Nat. Prod. 2011, 74, 1313–1317.




