Asymmetric Synthesis of Chiral Aliphatic α-Tertiary Aminonitriles via Organocatalytic Isomerization of Cyanoketimines
Ling Xu
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310027 China
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorCorresponding Author
Jisheng Luo
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
E-mails: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Li Deng
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang, 310030 China
E-mails: [email protected]; [email protected]Search for more papers by this authorLing Xu
Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310027 China
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Search for more papers by this authorCorresponding Author
Jisheng Luo
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
E-mails: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Li Deng
Key Laboratory of Precise Synthesis of Functional Molecules of Zhejiang Province, Department of Chemistry, School of Science, Westlake University, 600 Dunyu Road, Hangzhou, Zhejiang, 310030 China
Research Center for Industries of the Future, Westlake University, Hangzhou, Zhejiang, 310030 China
E-mails: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
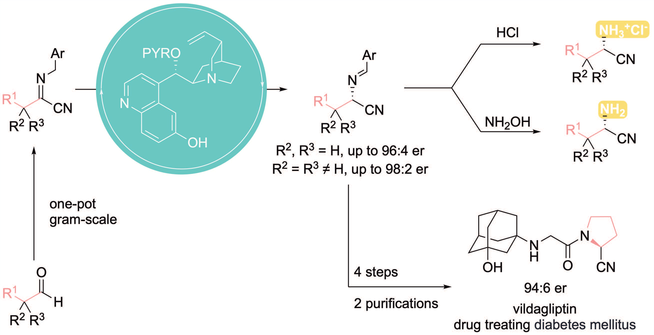
Chiral α-tertiary aminonitriles are valuable synthetic intermediates. They are also found in various structures of biologically active molecules. Therefore, numerous reports of catalytic asymmetric synthesis of chiral α-aminonitriles continuously emerged during the past few decades. Great strides have been made for the synthesis of chiral α-aryl and α-branched alkyl aminonitriles. However, efficient methods for catalytic asymmetric synthesis of chiral α-linear alkyl aminonitriles remain limited. We herein report a new synthetic approach to chiral α-tertiary alkyl aminonitriles via catalytic asymmetric isomerization of cyanoketimines. The synthetic value of this method was illustrated by application to a concise catalytic asymmetric synthesis of vildagliptin.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400774-sup-0001-supinfo.pdfPDF document, 8.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Enders, D.; Shilvock, J. P. Some Recent Applications of α-Aminonitrile Chemistry. Chem. Soc. Rev. 2000, 29, 359–373.
- 2 Grundke, C.; Vierengel, N.; Opatz, T. α-Aminonitriles: from Sustainable Preparation to Applications in Natural Product Synthesis. Chem. Rec. 2020, 20, 989–1016.
- 3 Gao, A.; Hamada, T.; Snyder, S. A. The Enantioselective Total Synthesis of Exochomine. Angew. Chem. Int. Ed. 2016, 55, 10301–10306.
- 4 Lebold, T. P.; Wood, J. L.; Deitch, J.; Lodewyk, M. W.; Tantillo, D. J.; Sarpong, R. A Divergent Approach to the Synthesis of the Yohimbinoid Alkaloids Venenatine and Alstovenine. Nat. Chem. 2013, 5, 126–131.
- 5 Lim, W.; Rhee, Y. H. A Concise Synthetic Method towards (–)-Swainsonine and Its 8-Epimer by Using Palladium-catalyzed Asymmetric Hydroamination of Alkoxyallene as the Key Strategy. Tetrahedron 2015, 71, 5939–5945.
- 6 Kouznetsov, V. V.; Galvis, C. E. P. Strecker Reaction and α-Amino Nitriles: Recent Advances in Their Chemistry, Synthesis, and Biological Properties. Tetrahedron 2018, 74, 773–810.
- 7 Fleming, F.; Yao, L.; Ravikumar, P.; Funk, L.; Shook, B. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917.
- 8 Chio, C. D.; Previti, S.; Amendola, G.; Ravichandran, R.; Wagner, A.; Cosconati, S.; Hellmich, U. A.; Schirmeister, T.; Zappalà, M.; Ettari, R. Development of Novel Dipeptide Nitriles as Inhibitors of Rhodesain of Trypanosoma Brucei Rhodesiense. Eur. J. Med. Chem. 2022, 236, 114328.
- 9 Ward, Y. D.; Thomson, D. S.; Frye, L. L.; Cywin, C. L.; Morwick, T.; Emmanuel, M. J.; Zindell, R.; McNeil, D.; Bekkali, Y.; Girardot, M.; Hrapchak, M.; DeTuri, M.; Crane, K.; White, D.; Pav, S.; Wang, Y.; Hao, M. H.; Grygon, C. A.; Labadia, M. E.; Freeman, D. M.; Davidson, W.; Hopkins, J. L.; Brown, M. L.; Spero, D. M. Design and Synthesis of Dipeptide Nitriles as Reversible and Potent Cathepsin S Inhibitors. J. Med. Chem. 2002, 45, 5471–5482.
- 10 Schmitz, J.; Li, T.; Bartz, U.; Gütschow, M. Cathepsin B inhibitors: Combining Dipeptide Ntriles with an Occluding Loop Recognition Element by Click Chemistry. ACS Med. Chem. Lett. 2016, 7, 211–216.
- 11 Bonatto, V.; Lameiro, R. F.; Rocho, F. R.; Lameira, J.; Leitão, A.; Montanari, C. A. Nitriles: An Attractive Approach to the Development of Covalent Inhibitors. RSC Med. Chem. 2023, 14, 201–207.
- 12 Fleming, F. F. Nitrile-containing Natural Products. Nat. Prod. Rep. 1999, 16, 597–606.
- 13 Hughes, T. E.; Mone, M. D.; Russell, M. E.; Weldon, S. C.; Villhauer, E. B. NVP-DPP728 (1-[[[2-[(5-Cyanopyridin-2-yl)amino]ethyl]amino]acetyl]-2-cyano-(S)-pyrrolidine), a Slow-binding Inhibitor of Dipeptidyl Peptidase IV. Biochemistry 1999, 38, 11597–11603.
- 14 Owen, D. R.; Allerton, C. M.; Anderson, A. S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R. D.; Carlo, A.; Coffman, K. J.; Dantonio, A.; Di, L.; Eng, H.; Ferre, R.; Gajiwala, K. S.; Gibson, S.; Greasley, S. E.; Hurst, B. L.; Kadar, E. P.; Kalgutkar, A. S.; Lee, J. C.; Lee, J.; Liu, W.; Mason, S. W.; Noell, S.; Novak, J. J.; Obach, R. S.; Ogilvie, K.; Patel, N. C.; Pettersson, M.; Rai, D. K.; Reese, M. R.; Sammons, M. F.; Sathish, J. G.; Singh, R. S. P.; Steppan, C. M.; Stewart, A. E.; Tuttle, J. B.; Updyke, L.; Verhoest, P. R.; Wei, L.; Yang, Q.; Zhu, Y. An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19. Science 2021, 374, 1586–1593.
- 15 Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V. M.; Damle, B.; Simón-Campos, A.; Pypstra, R.; Rusnak, J. M. Oral Nirmatrelvir for High-risk, Nonhospitalized Adults with Covid-19. New Engl. J. Med. 2022, 386, 1397–1408.
- 16 Juillerat-Jeanneret, L. Dipeptidyl Peptidase IV and Its Inhibitors: Therapeutics for Type 2 Diabetes and What Else? J. Med. Chem. 2014, 57, 2197–2212.
- 17 Augeri, D. J.; Robl, J. A.; Betebenner, D. A.; Magnin, D. R.; Khanna, A.; Robertson, J. G.; Wang, A.; Simpkins, L. M.; Taunk, P.; Huang, Q.; Han, S.; Abboa-Offei, B.; Cap, M.; Xin, L.; Tao, L.; Tozzo, E.; Welzel, G. E.; Egan, D. M.; Marcinkeviciene, J.; Chang, S. Y.; Biller, S. A.; Kirby, M. S.; Parker, R. A.; Hamann, L. G. Discovery and Preclinical Profile of Saxagliptin (BMS-477118): A Highly Potent, Long-acting, Orally Active Dipeptidyl Peptidase IV Inhibitor for the Treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 5025–5037.
- 18
Cai, X.; Xie, B. Recent Advances in Asymmetric Strecker Reactions. ARKIVOC 2014, I, 205–248.
10.3998/ark.5550190.p008.487 Google Scholar
- 19 Liu, Y.; Zhou, J. Catalytic Asymmetric Strecker Reaction: Bifunctional Chiral Tertiary Amine/Hydrogen-Bond, Donor Catalysis Joins the Field. Synthesis 2015, 47, 1210–1226.
- 20 Gröger, H. Catalytic Enantioselective Strecker Reactions and Analogous Syntheses. Chem. Rev. 2003, 103, 2795–2827.
- 21 Wu, W.; Yu, J.; Zhou, J. Catalytic Enantioselective Cyanation: Recent Advances and Perspectives. ACS Catal. 2020, 10, 7668−7690.
- 22 Kurono, N.; Ohkuma, T. Catalytic Asymmetric Cyanation Reactions. ACS Catal. 2016, 6, 989−1023.
- 23 Merino, P.; Marqués-López, E.; Tejero, T.; Herrera, R. P. Organocatalyzed Strecker Reactions. Tetrahedron 2009, 65, 1219–1234.
- 24 Wang, J.; Liu, X.; Feng, X. Asymmetric Strecker Reactions. Chem. Rev. 2011, 111, 6947–6983.
- 25 Vachal, P.; Jacobsen, E. N. Structure-based Analysis and Optimization of a Highly Enantioselective Catalyst for the Strecker Reaction. J. Am. Chem. Soc. 2002, 124, 10012–10014.
- 26 Ooi, T.; Uematsu, Y.; Fujimoto, J.; Fukumoto, K.; Maruoka, K. Advantage of In Situ Generation of N-Arylsulfonyl Imines from α-Amide Sulfones in the Phase-transfer-catalyzed Asymmetric Strecker Reaction. Tetrahedron Lett. 2007, 48, 1337–1340.
- 27 Ishitani, H.; Komiyama, S.; Hasegawa, Y.; Kobayashi, S. Catalytic Asymmetric Strecker Synthesis. Preparation of Enantiomerically Pure α-Amino Acid Derivatives from Aldimines and Tributyltin Cyanide or Achiral Aldehydes, Amines, and Hydrogen Cyanide Using a Chiral Zirconium Catalyst. J. Am. Chem. Soc. 2000, 122, 762–766.
- 28 Wu, Y.; Singh, R. P.; Deng, L. Asymmetric Olefin Isomerization of Butenolides via Proton Transfer Catalysis by an Organic Molecule. J. Am. Chem. Soc. 2011, 133, 12458–12461.
- 29
Zeng, Y.; Fei, C.; Zhou, X.; Deng, L. Chiral Betaine-mediated Efficient Organocatalytic Asymmetric Isomerization of β,γ-Unsaturated Butenolides. Synlett 2023, 20, 2429–2432.
10.1055/s-0042-1751496 Google Scholar
- 30 Zhou, X.; Wu, Y.; Deng, L. Cinchonium Betaines as Efficient Catalysts for Asymmetric Proton Transfer Catalysis: The Development of a Practical Enantioselective Isomerization of Trifluoromethyl Imines. J. Am. Chem. Soc. 2016, 138, 12297−12302.
- 31 Wu, Y.; Deng, L. Asymmetric Synthesis of Trifluoromethylated Amines via Catalytic Enantioselective Isomerization of Imines. J. Am. Chem. Soc. 2012, 134, 14334−14337.
- 32 Xiao, X.; Xie, Y.; Su, C.; Liu, M.; Shi, Y. Organocatalytic Asymmetric Biomimetic Transamination: From α-Keto Esters to Optically Active α-Amino Acid Derivatives. J. Am. Chem. Soc. 2011, 133, 12914–12917.
- 33 Li, H.; Wang, Y.; Tang, L.; Wu, F.; Liu, X.; Guo, C.; Foxman, B. M.; Deng, L. Stereocontrolled Creation of Adjacent Quaternary and Tertiary Stereocenters by a Catalytic Conjugate Addition. Angew. Chem. Int. Ed. 2005, 44, 105–108.
- 34 Wang, Y.; Liu, X.; Deng, L. Dual-function Cinchona Alkaloid Catalysis: Catalytic Asymmetric Tandem Conjugate Addition-protonation for the Direct Creation of Nonadjacent Stereocenters. J. Am. Chem. Soc. 2006, 128, 3928–3930.
- 35 Li, H.; Song, J.; Deng, L. Catalytic Enantioselective Conjugate Additions with α,β-Unsaturated Sulfones. Tetrahedron 2009, 65, 3139–3148.
- 36The absolute configuration was confirmed as S according to the following literature: Herrera, R. P.; Sgarzani, V.; Bernardi, L.; Fini, F.; Pettersen, D.; Ricci, A. Phase Transfer Catalyzed Enantioselective Strecker Reactions of α-Amido Sulfones with Cyanohydrins. J. Org. Chem. 2006, 71, 9869–9872.
- 37Room temperature purification by flash chromatography on silica gel might cause hydrolysis of the aminonitriles. Low temperature purification was highly recommended. Alternatively, both of the eluents were cooled by NaCl-ice bath before purification.




