Synthesis of Indole-Fused Eight-Membered Oxa-Rings via Palladium-Catalyzed Asymmetric [4+4] Cycloaddition of Indole-2,3-Quinodimethanes with 2-Alkylidenetrimethylene Carbonates
Xinhao Lu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorYi Wang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
These authors contributed equally to this work.
Search for more papers by this authorChenghan He
School of Science and Engineering, The Chinese University of Hong Kong, Shenzhen, Guangdong, 518172 China
Search for more papers by this authorCorresponding Author
Yang-Zi Liu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei-Ping Deng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXinhao Lu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
These authors contributed equally to this work.
Search for more papers by this authorYi Wang
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
These authors contributed equally to this work.
Search for more papers by this authorChenghan He
School of Science and Engineering, The Chinese University of Hong Kong, Shenzhen, Guangdong, 518172 China
Search for more papers by this authorCorresponding Author
Yang-Zi Liu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei-Ping Deng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, Jinhua, Zhejiang, 321004 China
Shanghai Frontiers Science Center of Optogenetic Techniques for Cell Metabolism, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
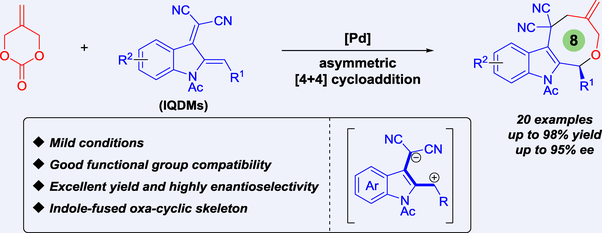
We report a palladium-catalyzed asymmetric [4+4] cycloaddition reaction between 2-alkylidenetrimethylene carbonate and electron-deficient indole-2,3-quinodimethanes (IQDMs). This reaction features exclusive regioselectivity, high yield (up to 98%), excellent enantioselectivity (up to 95% ee), and easy scale-up without any loss of efficiency, making it valuable for the synthesis of indole-fused eight-membered oxa-rings.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400764-sup-0001-supinfo.pdfPDF document, 5.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Pawelka K. H.; Stöckigt, J. Indole Alkaloids from Ochrosia Elliptica Plant Cell Suspension Cultures. Z. Naturforsch. C 1986, 41, 381–384; (b) Pawelka, K. H.; Stöckigt, J.; Danieli, B. Epchrosine – A New Indole Alkaloid Isolated from Plant Cell Cultures of Ochrosia Elliptica Labill. Plant Cell Rep. 1986, 5, 147–149; (c) Zhang, B.-J.; Yan, J.-M.; Wu, Z.-K.; Liu, Y.-P.; Bao, M.-F.; Cheng, G.-G.; Luo, X.-D.; Cai, X.-H.; Li, Y. Alkaloids from Ochrosia Borbonica. Helv. Chim. Acta 2013, 96, 2288–2298; (d) Stukenbrock, H.; Mussmann, R.; Geese, M.; Ferandin, Y.; Lozach, O.; Lemcke, T.; Kegel, S.; Lomow, A.; Burk, U.; Dohrmann, C.; Meijer, L.; Austen, M.; Kunick, C. 9-Cyano-1-Azapaullone (Cazpaullone), a Glycogen Synthase Kinase-3 (GSK-3) Inhibitor Activating Pancreatic β Cell Protection and Replication. J. Med. Chem. 2008, 51, 2196–2207; (e) Yamaguchi, A.; Inuki, S.; Ohta, K.; Oishi, S.; Asai, A.; Ohno, H. Identification of a Novel Indoleamine 2,3-Dioxygenase Inhibitor Bearing an Eight-Membered Ring Fused Indole Scaffold and Its Structure-Activity Relationship. Heterocycles 2021, 103, 331–347; (f) Hesse, R.; Gruner, K. K.; Kataeva, O.; Schmidt, A. W.; Knölker, H. J. Efficient Construction of Pyrano[3,2-a]carbazoles: Application to a Biomimetic Total Synthesis of Cyclized Monoterpenoid Pyrano[3,2- a]carbazole Alkaloids. Chem. Eur. J. 2013, 19, 14098–14111; (g) Delgado, A.; Clardy, J. Total Synthesis of (-)-Ovatolide. J. Org. Chem. 1993, 58, 2862–2866.
- 2For selected recent examples, see: (a) Hamada, N.; Yoshida, Y.; Oishi, S.; Ohno, H. Gold-Catalyzed Cascade Reaction of Skipped Diynes for the Construction of a Cyclohepta[b]pyrrole Scaffold. Org. Lett. 2017, 19, 3875–3878; (b) Wang, Z.; Addepalli, Y.; He, Y. Construction of Polycyclic Indole Derivatives via Multiple Aryne Reactions with Azaheptafulvenes. Org. Lett. 2018, 20, 644–647; (c) Tymann, D.; Tymann, D. C.; Bednarzick, U.; Iovkova-Berends, L.; Rehbein, J.; Hiersemann, M. Development of an Alkyne Analogue of the de Mayo Reaction: Synthesis of Medium-Sized Carbacycles and Cyclohepta[b]indoles. Angew. Chem. Int. Ed. 2018, 57, 15553–15557; (d) Kaufmann, J.; Jäckel, E.; Haak, E. Ruthenium-Catalyzed Cascade Annulation of Indole with Propargyl Alcohols. Angew. Chem. Int. Ed. 2018, 57, 5908–5911; (e) Jadhav, A. S.; Pankhade, Y. A.; Vijaya Anand, R. Exploring Gold Catalysis in a 1,6-Conjugate Addition/Domino Electrophilic Cyclization Cascade: Synthesis of Cyclohepta[b]indoles. J. Org. Chem. 2018, 83, 8615–8626; (f) Zeng, Q.; Dong, K.; Huang, J.; Qiu, L.; Xu, X. Copper-Catalyzed Carbene/Alkyne Metathesis Terminated with the Buchner Reaction: Synthesis of Dihydrocyclohepta[b]indoles. Org. Biomol. Chem. 2019, 17, 2326–2330.
- 3(a) Yuan, Y.; Guo, X.; Zhang, X.; Li, B.; Huang, Q. Access to 5H-Benzo[a]carbazol-6-ols and Benzo[6,7]cyclohepta[1,2-b]indol-6- ols via Rhodium-Catalyzed C-H Activation/Carbenoid Insertion/Aldol- Type Cyclization. Org. Chem. Front. 2020, 7, 3146–3159; (b) Tymann, D. C.; Benedix, L.; Iovkova, L.; Pallach, R.; Henke, S.; Tymann, D.; Hiersemann, M. Photochemical Approach to the Cyclohepta[b]indole Scaffold by Annulative Two-Carbon Ring-Expansion. Chem. Eur. J. 2020, 26, 11974–11978; (c) Mu, X.-P.; Li, Y.-H.; Zheng, N.; Long, J.-Y.; Chen, S.-J.; Liu, B.-Y.; Zhao, C.-B.; Yang, Z. Stereoselective Synthesis of Cyclohepta[b]indoles by Visible-Light-Induced [2+2]-Cycloaddition/Retro-Mannich-Type Reactions. Angew. Chem. Int. Ed. 2021, 60, 11211–11216; (d) Dhawa, U.; Connon, R.; Oliveira, J. C.; Steinbock, R.; Ackermann, L. Enantioselective Ruthenium-Catalyzed C-H Alkylations by a Chiral Carboxylic Acid with Attractive Dispersive Interactions. Org. Lett. 2021, 23, 2760–2765; (e) Sun, Y.; Wang, Z.; Wu, S.; Zhang, Y.; Shi, F. Lewis Acid-Catalyzed [4+2] Cycloaddition of 3-Alkyl-2-Vinylindoles with β,γ-Unsaturated α-Ketoesters. Green Synth. Catal. 2022, 3, 84–88.
- 4(a) Modha, S. G.; Vachhani, D. D.; Jacobs, J.; Van Meervelt, L.; Van der Eycken, E. V. A Concise Route to Indoloazocines via a Sequential Ugi-Gold-Catalyzed Intramolecular Hydroarylation. Chem. Commun. 2012, 48, 6550–6552; (b) Kumar, A.; Li, Z.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Switching the Regioselectivity via Indium(III) and Gold(I) Catalysis: A Post-Ugi Intramolecular Hydroarylation to Azepino-and Azocino-[c,d]indolones. Chem. Commun. 2013, 49, 6803–6805; (c) Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Diversely Substituted Indoloazepinones and Indoloazocinones: A Post-Ugi Gold-Catalyzed Regioselective Carbocyclization Approach. Synthesis 2015, 47, 1337–1347; (d) Yang, J.-M.; Li, P.-H.; Wei, Y.; Tang, X.-Y.; Shi, M. Gold(I)-Catalyzed Highly Stereoselective Synthesis of Polycyclic Indolines: The Construction of Four Contiguous Stereocenters. Chem. Commun. 2016, 52, 346–349; (e) Tan, T.-D.; Zhu, X.-Q.; Jia, M.; Lin, Y.; Cheng, J.; Xia, Y.; Ye, L.-W. Stereospecific Access to Bridged [n.2.1] Skeletons Through Gold-Catalyzed Tandem Reaction of Indolyl Homopropargyl Amides. Chin. Chem. Lett. 2020, 31, 1309–1312.
- 5(a) Zhang, M.-M.; Qu, B.-L.; Shi, B.; Xiao, W.-J.; Lu, L.-Q. High-Order Dipolar Annulations with Metal-Containing Reactive Dipoles. Chem. Soc. Rev. 2022, 51, 4146–4174; (b) Zhang, J.; Chen, Y.; Wang, Q.; Shen, J.; Liu, Y.-Z.; Deng, W.-P. Transition Metal-Catalyzed Asymmetric Cyclizations Involving Allyl or Propargyl Heteroatom-Dipole Precursors. Chin. J. Org. Chem. 2022, 42, 3051–3101.
- 6(a) Cheng, B.; Volpin, G.; Morstein, J.; Trauner, D. Total Synthesis of (±)–Exotine B. Org. Lett. 2018, 20, 4358–4361; (b) Li, L.; Liu, T.; Ren, W.; Wang, Y. Catalyst-Free and Atom-Economical [4+3] Cycloaddition of Azadienes with Cyclic Azomethine Imines for Facile Synthesis of 1,2,4-Triazepines. Green Synth. Catal. 2024, 5, 57–67.
- 7(a) Takeda, T.; Harada, S.; Okabe, A.; Nishida, A. Cyclohepta[b]indole Synthesis through [5+2] Cycloaddition: Bifunctional Indium(III)-Catalyzed Stereoselective Construction of 7-Membered Ring Fused Indoles. J. Org. Chem. 2018, 83, 11541–11551; (b) Parker, A. N.; Martin, M. C.; Shenje, R.; France, S. Calcium-Catalyzed Formal [5+2] Cycloadditions of Alkylidene β-Ketoesters with Olefins: Chemodivergent Synthesis of Highly Functionalized Cyclohepta[b]indole Derivatives. Org. Lett. 2019, 21, 7268–7273.
- 8 Huang, L.; Dai, L.-X.; You, S.-L. Enantioselective Synthesis of Indole- Annulated Medium-Sized Rings. J. Am. Chem. Soc. 2016, 138, 5793–5796.
- 9(a) Zhu, C.; Wang, D.; Zhao, Y.; Sun, W.-Y.; Shi, Z. Enantioselective Palladium-Catalyzed Intramolecular α-Arylative Desymmetrization of 1,3-Diketones. J. Am. Chem. Soc. 2017, 139, 16486–16489; (b) Gao, X.; Xia, M.; Yuan, C.; Zhou, L.; Sun, W.; Li, C.; Wu, B.; Zhu, D.; Zhang, C.; Zheng, B.; Wang, D.; Guo, H. Enantioselective Synthesis of Chiral Medium-Sized Cyclic Compounds via Tandem Cycloaddition/Cope Rearrangement Strategy. ACS Catal. 2019, 9, 1645–1654; (c) Cheng, Q.; Xie, J.-H.; Weng, Y.-C.; You, S.-L. Pd-Catalyzed Dearomatization of Anthranils with Vinylcyclopropanes by [4+3] Cyclization Reaction. Angew. Chem. Int. Ed. 2019, 58, 5739–5743.
- 10 Kang, G.; Yamagami, M.; Vellalath, S.; Romo, D. Enantioselective Synthesis of Medium-Sized Lactams via Chiral α,β-Unsaturated Acylammonium Salts. Angew. Chem. Int. Ed. 2018, 57, 6527–6531.
- 11 Wei, Y.; Liu, S.; Li, M.-M.; Li, Y.; Lan, Y.; Lu, L.-Q.; Xiao, W.-J. Enantioselective Trapping of Pd-Containing 1,5-Dipoles by Photogenerated Ketenes: Access to 7-Membered Lactones Bearing Chiral Quaternary Stereocenters. J. Am. Chem. Soc. 2018, 141, 133–137.
- 12For selected reviews, see: (a) Lautens, M.; Klute, W.; Tam, W. Transition Metal-Mediated Cycloaddition Reactions. Chem. Rev. 1996, 96, 49–92; (b) Yet, L. Metal-Mediated Synthesis of Medium-Sized Rings. Chem. Rev. 2000, 100, 2963–3008; (c) Inglesby, P. A.; Evans, P. A. Stereoselective Rransition Metal-Catalysed Higher-Order Carbocyclisation Reactions. Chem. Soc. Rev. 2010, 39, 2791–2805; (d) Yang, S.; Shi, M. Recent Advances in Transition-Metal-Catalyzed/Mediated Transformations of Vinylidenecyclopropanes. Acc. Chem. Res. 2018, 51, 1667–1680; (e) Trost, B. M.; Mata, G. Forging Odd-Membered Rings: Palladium-Catalyzed Asymmetric Cycloadditions of Trimethylenemethane. Acc. Chem. Res. 2020, 53, 1293–1305; (f) Wang, J.; Blaszczyk, S. A.; Li, X.; Tang, W. Transition Metal-Catalyzed Selective Carbon-Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions. Chem. Rev. 2020, 121, 110–139.
- 13 Chen, J.-R.; Hu, X.-Q.; Xiao, W.-J. Metal-Containing Carbonyl Ylides: Versatile Reactants in Catalytic Enantioselective Cascade Reactions. Angew. Chem. Int. Ed. 2014, 53, 4038–4040.
- 14(a) Li, Q.; Pan, R.; Wang, M.; Yao, H.; Lin, A. Ligand-Controlled, Palladium-Catalyzed Asymmetric [4+4] and [2+4] Cycloadditions. Org. Lett. 2021, 23, 2292–2297; (b) Chen, Y.; Zang, M.; Wang, W.; Liu, Y.-Z.; Luo, X.; Deng, W.-P. Palladium-Catalyzed [4+4] Cycloaddition of Homo-TMM All-Carbon 1,4-Dipole Precursors for Construction of Benzofuro[3,2-b]azocines and Furo[3,2-b]azocines, Chin. J. Chem. 2023, 41, 2825–2831; (c) Chen, G.-H.; Ye, Y.; Zhang, D.-X.; Li, H.-J.; Zhang, N.; Liang, G.-J.; Zhang, D.; Zhou, J.; Zhou, H. Pd-Catalyzed Enantioselective [4+4] Dipolar Cycloaddition of Aliphatic 1,4-Dipoles with Azadienes to Access Eight-Membered N-Heterocycles. Org. Chem. Front. 2023, 10, 4698–4702.
- 15 Shintani, R.; Moriya, K.; Hayashi, T. Guiding the Nitrogen Nucleophile to the Middle: Palladium-Catalyzed Decarboxylative Cyclopropanation of 2-Alkylidenetrimethylene Carbonates with Isocyanates. Chem. Commun. 2011, 47, 3057–3059.
- 16 Mao, B.; Liu, H.; Yan, Z.; Xu, Y.; Xu, J.; Wang, W.; Wu, Y.; Guo, H. Palladium-Catalyzed Asymmetric [4+2] Cycloaddition of 2-Methylidenetrimethylene Carbonate with Alkenes: Access to Chiral Tetrahydropyran-Fused Spirocyclic Scaffolds. Angew. Chem. Int. Ed. 2020, 59, 11316–11320.
- 17 Li, M.-M.; Qu, B.-L.; Xiao, Y.-Q.; Xiao, W.-J.; Lu, L.-Q. Enantioselective Trapping of Palladium-Stabilized Oxo-1,4-Dipoles with Photochemically Generated Ketenes. Sci. Bull. 2021, 66, 1719–1722.
- 18 Dou, P.-H.; Yuan, S.-P.; Chen, Y.; Zhao, J.-Q.; Wang, Z.-H.; You, Y.; Zhang, Y.-P.; Zhou, M. Q.; Yuan, W. C. Dearomatization of 3-Nitroindoles Enabled Using Palladium-Catalyzed Decarboxylative [4+2] Cycloaddition of 2-Alkylidenetrimethylene Carbonates. J. Org. Chem. 2022, 87, 6025–6037.
- 19
Uno, H.; Kawai, K.; Araki, T.; Shiro, M.; Shibata, N. Enantio-, Diastereo- and Regioselective Synthesis of Chiral Cyclic and Acyclic gem-Difluoromethylenes by Palladium-Catalyzed [4+2] Cycloaddition. Angew. Chem. Int. Ed. 2022, 134, e202117635.
10.1002/ange.202117635 Google Scholar
- 20 Uno, H.; Kawai, K.; Shiro, M.; Shibata, N. Modular Synthesis of Medium-Sized Fluorinated and Nonfluorinated Heterocyclic Lactones by Sequential CN-Bond-Cleaving Ring Expansion under Pd Catalysis. ACS Catal. 2020, 10, 14117–14126.
- 21 Li, Y.; Jie, J.; Li, H.; Yang, H.; Fu, H. Synthesis of Spirotetrahydrofuran Oxindoles via Palladium-Catalyzed [4+1] Cycloaddition of Diphenyl 2-Oxoindolin-3-yl Phosphates and 2-Methylidenetrimethylene Carbonate. Org. Lett. 2021, 23, 6499–6503.
- 22 Chen, Z.; Chen, Z.-C.; Du, W.; Chen, Y.-C. Asymmetric [4+3] Annulations for Constructing Divergent Oxepane Frameworks via Cooperative Tertiary Amine/Transition Metal Catalysis. Org. Lett. 2021, 23, 8559–8564.
- 23 Meng, Y.; Song, M.; Wang, Y.; Wang, Y.; Li, E.-Q. Palladium-Catalyzed Asymmetric (4+3) Cycloaddition of N-2,2,2-Trifluoroethylisatin Ketimines: Access to Optically Active Spirooxindoles. Org. Chem. Front. 2023, 10, 2648–2652.
- 24 Xie, H.; Chen, L.; Han, Z.; Yang, Z.; Sun, J.; Huang, H. Pd-Catalyzed Ligand-Directed Divergent Cycloaddition of Cyclic 1-Azadienes with Oxo-1,4-dipoles. Org. Lett. 2023, 25, 5011–5016.
- 25 Lin, H.; Mao, B.; Han, B.; Luo, J.; Ge, Y.; Zhang, X.; Wang, C.; Guo, H.; Yuan, C. Palladium-Catalyzed [4+4] Cycloaddition of 2-Pyrones with 2-Alkylidenetrimethylene Carbonates: Access to Bridged Eight-Membered Oxygen Heterocycles. Org. Chem. Front. 2024, 11, 2864–2869.
- 26 Chen, L.; Xie, H.; Xue, Y.; Han, Z.; Sun, J.; Huang, H. Palladium-Catalyzed [4+2] and [6+2] Dipolar Cycloadditions for the Construction of Benzo[d]isothiazole 1,1-Dioxide Fused 1,3-Oxazinanes and 1,3-Oxazocanes. Chin. J. Chem. 2024, 42, 829–834.
- 27For selected reviews, see: (a) Guo, W.; Gómez, J. E.; Cristòfol, À.; Xie, J.; Kleij, A. W. Catalytic Transformations of Functionalized Cyclic Organic Carbonates. Angew. Chem. Int. Ed. 2018, 57, 13735–13747; (b) Zuo, L.; Liu, T.; Chang, X.; Guo, W. An Update of Transition Metal-Catalyzed Decarboxylative Transformations of Cyclic Carbonates and Carbamates. Molecules 2019, 24, 3930; (c) Cho, H. J.; Kim, J. H. Pd-Catalyzed Oxa-[4+n] Dipolar Cycloaddition Using 1,4-O/C Dipole Synthons for the Synthesis of O-Heterocycles. Org. Biomol. Chem. 2023, 21, 9507–9518.
- 28 Gu, B.-Q.; Yang, W.-L.; Wu, S.-X.; Wang, Y.-B.; Deng, W.-P. Organocatalytic Asymmetric Synthesis of Tetrahydrocarbazoles via an Inverse-Electron-Demand Diels-Alder Reaction of 2,3-Indole-Dienes with Enals. Org. Chem. Front. 2018, 5, 3430–3434.
- 29 Zheng, X.; Sun, H.; Yang, W.-L.; Deng, W.-P. Elaboration of Phosphoramidite Ligands Enabling Palladium-Catalyzed Diastereo- and Enantioselective All Carbon [4+3] Cycloaddition. Sci. China Chem. 2020, 63, 911–916.
- 30 Wang, Y.; Zhang, J.; Liu, Y.; Luo, X.; Deng, W.-P. Palladium-Catalyzed Asymmetric [3+4] Cycloadditions for the Construction of Cyclohepta[b]indoles. Chin. J. Org. Chem. 2023, 43, 2864–2877.
- 31
Zheng, X.; Gao, Y.-S.; Mao, J.; Wu, S.-X.; Yang, W.-L.; Luo, X.; Deng, W.-P. Organocatalytic Enantioselective [8+4] Cycloadditions of Isobenzofulvenes for the Construction of Bicyclo[4.2.1]nonanes. Chin. J. Chem. 2021, 39, 3219–3224.
10.1002/cjoc.202100250 Google Scholar
- 32 Linton, E. C.; Kozlowski, M. C. Catalytic Enantioselective Meerwein−Eschenmoser Claisen Rearrangement: Asymmetric Synthesis of Allyl Oxindoles. J. Am. Chem. Soc. 2008, 130, 16162–16163.




