Cobalt-Catalyzed Regioselective Methylative Coupling of Internal Alkynes with Aldehydes/Aldimines
Jiwu Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorZhikun Liang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Qinglei Chong
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Fanke Meng
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
Beijing National Laboratory for Molecular Sciences, Beijing, 100871 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJiwu Zhang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorZhikun Liang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Qinglei Chong
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Fanke Meng
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, 1 Sub-lane Xiangshan, Hangzhou, Zhejiang, 310024 China
Beijing National Laboratory for Molecular Sciences, Beijing, 100871 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
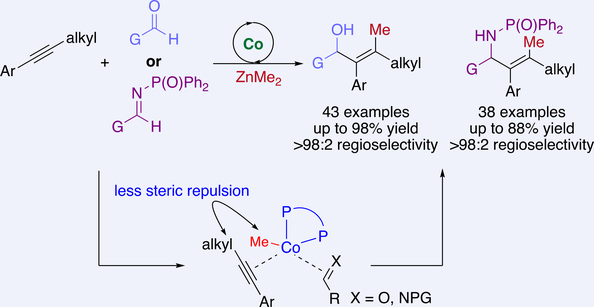
Catalytic methylative coupling of internal alkynes and aldehydes/aldimines through regioselective oxidative cyclization promoted by a phosphine–Co complex is presented. Such process constitutes an unprecedented and unique approach for Co-catalyzed generation of metallacycles that reversed inherent regiochemical biases to furnish a wide range of allylic alcohols and allylic amides bearing a tetrasubstituted alkene in up to 98% yield with high regioselectivity, representing a novel and general strategy for reversal of substrate-controlled regioselectivity in metal-catalyzed oxidative cyclization.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400641-sup-0001-supinfo.pdfPDF document, 12.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected recent reviews, see: (a) Holmes, M.; Schwartz, L. A.; Krische, M. J. Intermolecular Metal-Catalyzed Reductive Coupling of Dienes, Allenes, and Enynes with Carbonyl Compounds and Imines. Chem. Rev. 2018, 118, 6026–6052; (b) Ortiz, E.; Shezaf, J. Z.; Chang, Y.-H.; Krische, M. J. Enantioselective Metal-Catalyzed Reductive Coupling of Alkynes with Carbonyl Compounds and Imines: Convergent Construction of Allylic Alcohols and Amines. ACS Catal. 2022, 12, 8164–8174.
- 2 Huddleston, R. R.; Jang, H.-Y.; Krische, M. J. First Catalytic Reductive Coupling of 1,3-Diynes to Carbonyl Partners: A New Regio- and Enantioselective C–C Bond Forming Hydrogenation. J. Am. Chem. Soc. 2003, 125, 11488–11489.
- 3For selected examples, see: (a) Hong, Y.-T.; Cho, C.-W.; Skucas, E.; Krische, M. J. Enantioselective Reductive Coupling of 1,3-Enynes to Glyoxalates Mediated by Hydrogen: Asymmetric Synthesis of β,γ-Unsaturated α-Hydroxy Esters. Org. Lett. 2007, 9, 3745–3748; (b) Kong, J.-R.; Ngai, M.-Y.; Krische, M. J. Highly Enantioselective Direct Reductive Coupling of Conjugated Alkynes and α-Ketoesters via Rhodium-Catalyzed Asymmetric Hydrogenation. J. Am. Chem. Soc. 2006, 128, 718–719; (c) Komanduri, V.; Krische, M. J. Enantioselective Reductive Coupling of 1,3-Enynes to Heterocyclic Aromatic Aldehydes and Ketones via Rhodium-Catalyzed Asymmetric Hydrogenation: Mechanistic Insight into the Role of Brønsted Acid Additives. J. Am. Chem. Soc. 2006, 128, 16448–16449; (d) Kong, J.-R.; Cho, C.-W.; Krische, M. J. Hydrogen-Mediated Reductive Coupling of Conjugated Alkynes with Ethyl (N-Sulfinyl)-iminoacetates: Synthesis of Unnatural α-Amino Acids via Rhodium-Catalyzed C–C Bond Forming Hydrogenation. J. Am. Chem. Soc. 2005, 127, 11269–11276; (e) Cho, C.-W.; Krische, M. J. Enantioselective Reductive Coupling of Alkynes and α-Keto Aldehydes via Rhodium-Catalyzed Hydrogenation: An Approach to Bryostatin Substructures. Org. Lett. 2006, 8, 891–894.
- 4For selected examples, see: (a) Skucas, E.; Kong, J.-R.; Krische, M. J. Enantioselective Reductive Coupling of Acetylene to N-Arylsulfonyl Imines via Rhodium Catalyzed C–C Bond Forming Hydrogenation: (Z)-Dienyl Allylic Amines. J. Am. Chem. Soc. 2007, 129, 7242–7243; (b) Kong, J.-R.; Krische, M. J. Catalytic Carbonyl (Z)-Dienylation via Multicomponent Reductive Coupling of Acetylene to Aldehydes and α- Ketoesters Mediated by Hydrogen: Carbonyl Insertion into Cationic Rhodacyclopentadienes. J. Am. Chem. Soc. 2006, 128, 16040–16041; (c) Barchuk, A.; Ngai, M.-Y.; Krische, M. J. Allylic Amines via Iridium Catalyzed C–C Bond Forming Hydrogenation: Imine Vinylation in the Absence of Stoichiometric Byproducts or Metallic Reagents. J. Am. Chem. Soc. 2007, 129, 8432–8433; (d) Ngai, M.-Y.; Barchuk, A.; Krische, M. J. Enantioselective Iridium Catalyzed Imine Vinylation: Optical Enriched Allylic Amines via Alkyne-Imine Reductive Coupling Mediated by Hydrogen. J. Am. Chem. Soc. 2007, 129, 12644–12645; (e) Patman, R. L.; Chaulagain, M. R.; Williams, V. M.; Krische, M. J. Direct Vinylation of Alcohols or Aldehydes Employing Alkynes as Vinyl Donors: A Ruthenium Catalyzed C–C Bond Forming Transfer Hydrogenation. J. Am. Chem. Soc. 2009, 131, 2066–2067; (f) Ortiz, E.; Chang, Y.-H.; Shezaf, J. Z.; Shen, W.; Krische, M. J. Stereo- and Site-Selective Conversion of Primary Alcohols to Allylic Alcohols via Ruthenium-Catalyzed Hydrogen Auto-Transfer Mediated by 2-Butyne. J. Am. Chem. Soc. 2022, 144, 8861–8869.
- 5For selected examples of Ni-catalyzed reductive coupling of aldehydes and alkynes, see: (a) Mahandru, G. M.; Liu, G.; Montgomery, J. Ligand-Dependent Scope and Divergent Mechanistic Behavior in Nickel-Catalyzed Reductive Couplings of Aldehydes and Alkynes. J. Am. Chem. Soc. 2004, 126, 3698–3699; (b) Chaulagain, M. R.; Sormunen, G. J.; Montgomery, J. New N-Heterocyclic Carbene Ligand and Its Application in Asymmetric Nickel-Catalyzed Aldehyde/Alkyne Reductive Couplings. J. Am. Chem. Soc. 2007, 129, 9568–9569; (c) Miller, K. M.; Huang, W.-S.; Jamison, T. F. Catalytic Asymmetric Reductive Coupling of Alkynes and Aldehydes: Enantioselective Synthesis of Allylic Alcohols and α-Hydroxy Ketones. J. Am. Chem. Soc. 2003, 125, 3442–3443; For instances of Ni-catalyzed alkylative coupling of alkynes and aldehydes/aldimines, see: (d) Nie, M.; Fu, W.; Cao, Z.; Tang, W. Enantioselective Nickel-Catalyzed Alkylative Alkyne-Aldehyde Cross-Coupling. Org. Chem. Front. 2015, 2, 1322–1325; (e) Yang, Y.; Zhu, S.-F.; Zhou, C.-Y.; Zhou, Q.-L. Nickel-Catalyzed Enantioselective Alkylative Coupling of Alkynes and Aldehydes: Synthesis of Chiral Allylic Alcohols with Tetrasubstituted Olefins. J. Am. Chem. Soc. 2008, 130, 14052–14053; (f) Patel, S. J.; Jamison, T. F. Catalytic Three- Component Coupling of Alkynes, Imines, and Organoboron Reagents. Angew. Chem. Int. Ed. 2003, 42, 1364–1367; (g) Patel, S. J.; Jamison, T. F. Asymmetric Catalytic Coupling of Organoboranes, Alkynes, and Imines with a Removable (Trialkylsilyloxy)ethyl Group-Direct Access to Enantiomerically Pure Primary Allylic Amines. Angew. Chem. Int. Ed. 2004, 43, 3941–3944; For Co-catalyzed reductive coupling of alkynes and aldehydes/aldimines, see: (h) Li, Y.-L.; Zhang, S.-Q.; Chen, J.; Xia, J.-B. Highly Regio- and Enantioselective Reductive Coupling of Alkynes and Aldehydes via Photoredox Cobalt Dual Catalysis. J. Am. Chem. Soc. 2021, 143, 7306–7313; (i) Bishop, H. D.; Zhao, Q.; Uyeda, C. Catalytic Asymmetric Synthesis of Zinc Metallacycles. J. Am. Chem. Soc. 2023, 145, 20152–20157; For Ni-catalyzed reductive coupling of alkynes and imines with an alcohol as a reductant, see: (j) Yao, W.-W.; Li, R.; Li, J.-F.; Sun, J.; Ye, M. NHC ligand-enabled Ni-catalyzed reductive coupling of alkynes and imines using isopropanol as a reductant. Green Chem. 2019, 21, 2240–2244; For selected recent examples of Ni-catalyzed enantioselective coupling of alkenes through oxidative cyclization, see: (k) Chen, Z.-H.; Gu, L.-J.; Wang, B.; Xiao, L.-J.; Ye, M.; Zhou, Q.-L. Regioselective and enantioselective nickel-catalyzed intermolecular reductive coupling of aliphatic alkenes with imines. J. Am. Chem. Soc. 2024, 146, 14915–14921; (l) Marcum, J. S.; Meek, S. J. Efficient enantio-, diastereo, E/Z-, and site-selective nickel-catalyzed fragment couplings of aldehydes, dienes, and organoborons. J. Am. Chem. Soc. 2022, 144, 19231–19237.
- 6 Zhou, C.-Y.; Zhu, S.-F.; Wang, L.-X.; Zhou, Q.-L. Enantioselective nickel-catalyzed reductive coupling of alkynes and imines. J. Am. Chem. Soc. 2010, 132, 10955–10957.
- 7(a) Wang, H.; Negretti, S.; Knauff, A. R.; Montgomery, J. Exo-Selective Reductive Macrocyclization of Ynals. Org. Lett. 2015, 17, 1493–1496; (b) Shareef, A.-R.; Sherman, D. H.; Montgomery, J. Nickel-Catalyzed Regiodivergent Approach to Macrolide Motifs. Chem. Sci. 2012, 3, 892–895; (c) Jackson, E. P.; Montgomery, J. Regiocontrol in Catalytic Reductive Couplings through Alterations of Silane Rate Dependence. J. Am. Chem. Soc. 2015, 137, 958–963; (d) Malik, H. A.; Sormunen, G. J.; Montgomery, J. A General Strategy for Regiocontrol in Nickel-Catalyzed Reductive Couplings of Aldehydes and Alkynes. J. Am. Chem. Soc. 2010, 132, 6304–6305; (e) Wang, H.; Lu, G.; Sormunen, G. J.; Malik, H. A.; Liu, P.; Montgomery, J. NHC Ligands Tailored for Simultaneous Regio- and Enantiocontrol in Nickel-Catalyzed Reductive Couplings. J. Am. Chem. Soc. 2017, 139, 9317–9324; (f) Jackson, E. P.; Malik, H. A.; Sormunen, G. J.; Baxter, R. D.; Liu, P.; Wang, H.; Shareef, A.-R.; Montgomery, J. Mechanistic Basis for Regioselection and Regiodivergence in Nickel-Catalyzed Reductive Couplings. Acc. Chem. Res. 2015, 48, 1736–1745.
- 8(a) Miller, K. M.; Jamison, T. F. Ligand-Switchable Directing Effects of Tethered Alkenes in Nickel-Catalyzed Additions to Alkynes. J. Am. Chem. Soc. 2004, 126, 15342–15343; (b) Moslin, R. M.; Moslin, K. M.; Jamison, T. F. Regioselectivity and Enantioselectivity in Nickel-Catalysed Reductive Coupling Reactions of Alkynes. Chem. Commun. 2007, 4441–4449.
- 9 Huang, W.; Bai, J.; Guo, Y.; Chong, Q.; Meng, F. Cobalt-Catalyzed Regiodivergent and Enantioselective Intermolecular Coupling of 1,1-Disubstituted Allenes and Aldehydes. Angew. Chem. Int. Ed. 2023, 62, e202219257.
- 10 Wang, H.; Jie, X.; Chong, Q.; Meng, F. Pathway-Divergent Coupling of 1,3-Enynes with Acrylates through Cascade Cobalt Catalysis. Nat. Commun. 2024, 15, 3427.
- 11 Davies, S. G.; Fletcher, A. M.; Thomson, J. E. Hydrogen Bond Directed Epoxidation: Diastereoselective Olefinic Oxidation of Allylic Alcohols and Amines. Org. Biomol. Chem. 2014, 12, 4544–4549.
- 12 Carlsen, P. H. J.; Katsuki, T.; Martin, V. S.; Sharpless, K. B. A Greatly Improved Procedure for Ruthenium Tetroxide Catalyzed Oxidations of Organic Compounds. J. Org. Chem. 1981, 46, 3936–3938.
- 13(a) Michigami, K.; Mita, T.; Sato, Y. Cobalt-Catalyzed Allylic C(sp3)–H Carboxylation with CO2. J. Am. Chem. Soc. 2017, 139, 6094–6097; (b) Mita, T.; Hanagata, S.; Michigami, K.; Sato, Y. Co-Catalyzed Direct Addition of Allylic C(sp3)–H Bonds to Ketones. Org. Lett. 2017, 19, 5876–5879; (c) Mita, T.; Uchiyama, M.; Michigami, K.; Sato, Y. Beilstein J. Org. Chem. 2018, 14, 2012–2017; (d) Zhang, H.; Huang, J.; Meng, F. Cobalt-Catalyzed Diastereo- and Enantioselective Allyl Addition to Aldehydes and α-Ketoesters through Allylic C–H Functionalization. Cell Rep. Phys. Sci. 2021, 2, 100406; (e) Mita, T.; Uchiyama, M.; Sato, Y. Catalytic Intramolecular Coupling of Ketoalkenes by Allylic C(sp3)–H Bond Cleavage: Synthesis of Five- and Six-Membered Carbocyclic Compounds. Adv. Synth. Catal. 2020, 362, 1275–1280.




