Photochemical Decarboxylative Radical Alkylation/Cyclization Reaction to Fused Nitrogen Heterocycles by LiI/PPh3 Catalysis
Jia-Li Sui
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorXin-Qian Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorShun-Dan Li
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Peng-Fei Huang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yu Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jin-Heng Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorJia-Li Sui
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorXin-Qian Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorShun-Dan Li
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
Search for more papers by this authorCorresponding Author
Peng-Fei Huang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yu Liu
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, Yueyang, Hunan, 414006 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jin-Heng Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemical Engineering, Qingdao University of Science and Technology, Qingdao, Shandong, 266042 China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
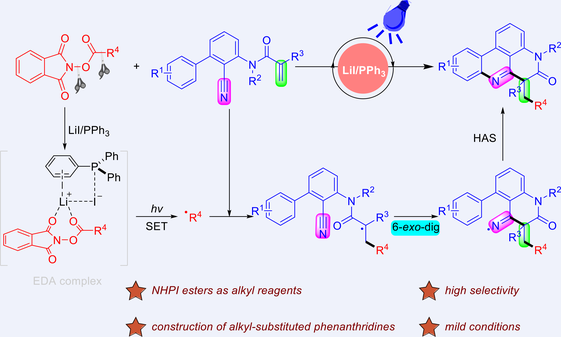
A visible-light-induced decarboxylative radical cascade cyclization reaction between N-(2-cyanoaryl)-acrylamides and alkyl N-(acyloxy)phthalimide (NHPI esters) for the construction of phenanthridine derivatives has been developed. This approach utilizes lithium iodide (LiI) and triphenylphosphine (PPh3) as the redox catalysts and the alkyl radical is produced through the photoactivation of the electron donor-acceptor (EDA) complex. A series of primary, secondary, and tertiary alkyl-substituted phenanthridines are prepared in up to 82% yield without transition-metal catalysts, chemical oxidants, or metal-/organic dye-based photocatalysts.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400628-sup-0001-supinfo.pdfPDF document, 11.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Li, Y.; Ge, L.; Muhammad, M. T.; Bao, H. Recent Progress on Radical Decarboxylative Alkylation for Csp3-C Bond Formation. Synthesis 2017, 49, 5263–5284; (b) Liang, Y.; Zhang, X.; MacMillan, D. W. C. Decarboxylative sp3 C-N Coupling via Dual Copper and Photoredox Catalysis. Nature 2018, 559, 83–88; (c) Patra, T.; Maiti, D. Decarboxylation as the Key Step in C-C Bond-Forming Reactions. Chem. - Eur. J. 2017, 23, 7382–7401; (d) Shao, T.; Ban, X.; Jiang, Z. α-Amino Acids: An Emerging Versatile Synthon in Visible Light-Driven Decarboxylative Transformations. Chem. Rec. 2023, 23, e202300122; (e) Wang, C.; Qiu, G.; Li, C.; Sun, K.; Wang, Z.; Wang, X. Heterocycle Synthesis via Decarboxylative Cyclization Methods. Adv. Synth. Catal. 2022, 364, 3756–3781; (f) Xiao, P.; Pannecoucke, X.; Bouillon, J.-P.; Couve-Bonnaire, S. Wonderful Fusion of Organofluorine Chemistry and Decarboxylation Strategy. Chem. Soc. Rev. 2021, 50, 6094–6151; (g) Zeng, Z.; Feceu, A.; Sivendran, N.; Gooßen, L. J. Decarboxylation-Initiated Intermolecular Carbon-Heteroatom Bond Formation. Adv. Synth. Catal. 2021, 363, 2678–2722.
- 2(a) Douthwaite, J. L.; Zhao, R.; Shim, E.; Mahjour, B.; Zimmerman, P. M.; Cernak, T. Formal Cross-Coupling of Amines and Carboxylic Acids to Form sp3-sp2 Carbon-Carbon Bonds. J. Am. Chem. Soc. 2023, 145, 10930–10937; (b) Kitcatt, D. M.; Nicolle, S.; Lee, A.-L. Direct Decarboxylative Giese Reactions. Chem. Soc. Rev. 2022, 51, 1415–1453; (c) Lai, X.-L.; Chen, M.; Wang, Y.; Song, J.; Xu, H.-C. Photoelectrochemical Asymmetric Catalysis Enables Direct and Enantioselective Decarboxylative Cyanation. J. Am. Chem. Soc. 2022, 144, 20201–20206; (d) Qi, X.-K.; Yao, L.-J.; Zheng, M.-J.; Zhao, L.; Yang, C.; Guo, L.; Xia, W. Photoinduced Hydrodifluoromethylation and Hydromethylation of Alkenes Enabled by Ligand-to-Iron Charge Transfer Mediated Decarboxylation. ACS Catal. 2024, 14, 1300–1310; (e) Rodríguez, N.; Goossen, L. J. Decarboxylative Coupling Reactions: a Modern Strategy for C-C-bond formation. Chem. Soc. Rev. 2011, 40, 5030–5048; (f) Xiong, N.; Li, Y.; Zeng, R. Merging Photoinduced Iron-Catalyzed Decarboxylation with Copper Catalysis for C-N and C-C Couplings. ACS Catal. 2023, 13, 1678–1685; (g) Xuan, J.; Zhang, Z.-G.; Xiao, W.-J. Visible-Light-Induced Decarboxylative Functionalization of Carboxylic Acids and Their Derivatives. Angew. Chem. Int. Ed. 2015, 54, 15632–15641.
- 3(a) Budnikov, A. S.; Krylov, I. B.; Lastovko, A. V.; Yu, B.; Terent'ev, A. O. N-Alkoxyphtalimides as Versatile Alkoxy Radical Precursors in Modern Organic Synthesis. Asian J. Org. Chem. 2022, 11, e202200262; (b) Murarka, S. N-(Acyloxy)phthalimides as Redox-Active Esters in Cross- Coupling Reactions. Adv. Synth. Catal. 2018, 360, 1735–1753; (c) Parida, S. K.; Hota, S. K.; Kumar, R.; Murarka, S. Late-Stage Alkylation of Heterocycles Using N-(Acyloxy)phthalimides. Chem. - Asian. J. 2021, 16, 879–889; (d) Parida, S. K.; Mandal, T.; Das, S.; Hota, S. K.; De Sarkar, S.; Murarka, S. Single Electron Transfer-Induced Redox Processes Involving N-(Acyloxy)phthalimides. ACS Catal. 2021, 11, 1640–1683; (e) Zhang, Y.; Ma, D.; Zhang, Z. Utilization of Photocatalysts in Decarboxylative Coupling of Carboxylic N-hydroxyphthalimide (NHPI) esters. Arabian J. Chem. 2022, 15, 103922.
- 4(a) Behnke, N. E.; Sales, Z. S.; Li, M.; Herrmann, A. T. Dual Photoredox/Nickel-Promoted Alkylation of Heteroaryl Halides with Redox- Active Esters. J. Org. Chem. 2021, 86, 12945–12955; (b) Cabrera- Afonso, M. J.; Sookezian, A.; Badir, S. O.; El Khatib, M.; Molander, G. A. Photoinduced 1,2-Dicarbofunctionalization of Alkenes with Organotrifluoroborate Nucleophiles via Radical/Polar Crossover. Chem. Sci. 2021, 12, 9189–9195; (c) Chen, P.; Huang, P.-F.; Xiong, B.-Q.; Huang, H.-W.; Tang, K.-W.; Liu, Y. Visible-Light-Induced Decarboxylative Alkylation/Ring Opening and Esterification of Vinylcyclopropanes. Org. Lett. 2022, 24, 5726–5730; (d) Liu, C.; Shen, N.; Shang, R. Photocatalytic Decarboxylative Alkylation of Silyl Enol Ether and Enamide with N-(acyloxy)phthalimide Using Ammonium Iodide. Org. Chem. Front. 2021, 8, 4166–4170; (e) Liu, Y.; Sui, J.-L.; Yu, W.-Q.; Xiong, B.-Q.; Tang, K.-W.; Zhong, L.-J. Visible-Light-Promoted Decarboxylative Alkylation/Cyclization of Vinylcycloalkanes. J. Org. Chem. 2023, 88, 8563–8575.
- 5(a) Guo, Y.; Luo, Y.; Mu, S.; Xu, J.; Song, Q. Photoinduced Decarboxylative Phosphorothiolation of N-Hydroxyphthalimide Esters. Org. Lett. 2021, 23, 6729–6734; (b) Hou, J.-Y.; Zhang, L.; He, S.-Y.; Ye, M.-L.; Chen, J.; Huang, T.-L.; Lv, G.-H.; Hai, L.; Yang, Z.-Z.; Wu, Y. Visible-Light-Induced Electron Donor-Acceptor (EDA) Complex-Initiated Synthesis of Non-Anomeric S-Aryl Glycosides. Org. Chem. Front. 2023, 10, 6200–6204; (c) Li, B.; Yi, L.; Maity, B.; Jia, J.; Shen, Y.; Chen, X.-Y.; Cavallo, L.; Rueping, M. Bio-inspired Halogen Bonding-Promoted Cross Coupling for the Synthesis of Organoselenium Compounds. ACS Catal. 2023, 13, 15194–15202; (d) Nagy, B.; Gonda, Z.; Földesi, T.; Fehér, P. P.; Stirling, A.; Tolnai, G. L.; Novák, Z. Photoinduced Decarboxylative Borylation of N-Hydroxyphthalimide Esters with Hypoboric Acid. Org. Lett. 2024, 26, 2292–2296; (e) Wang, R.; Xu, H.; Banerjee, A.; Cui, Z.; Ma, Y.; Whittingham, W. G.; Yang, P.; Li, A. Mild Approach to Nucleoside Analogues via Photoredox/Cu-Catalyzed Decarboxylative C-N Bond Formation. Total Synthesis of Oxetanocin A. Org. Lett. 2024, 26, 2691–2696; (f) Zhang, M.; Liu, L.; Tan, Y.; Jing, Y.; Liu, Y.; Wang, Z.; Wang, Q. Decarboxylative Radical Sulfilimination via Photoredox, Copper, and Brønsted Base Catalysis. Angew. Chem. Int. Ed. 2024, 63, e202318344.
- 6(a) Brals, J.; McGuire, T. M.; Watson, A. J. B. A Chemoselective Polarity-Mismatched Photocatalytic C(sp3)−C(sp2) Cross-Coupling Enabled by Synergistic Boron Activation. Angew. Chem. Int. Ed. 2023, 62, e202310462; (b) Mu, S.; Guo, Y.; Huang, X.; Luo, Y.; Chen, M.; Xu, J.; Song, Q. 4CzIPN-Catalyzed Radical-Initiated Cascade Cyclization for the Photosynthesis of Polysubstituted Quinolin-3-Amines. Org. Chem. Front. 2023, 10, 3259–3263; (c) Nie, Y.; Wang, Z.; Feng, Z.; Dong, B.; Bai, Y.; Leng, Y.; Wu, J. Na2-Eosin Y Catalyzed Alkylation of Enol Acetates by Radical Decarboxylation of N-Hydroxyphthalimide Esters. Asian J. Org. Chem. 2021, 10, 1675–1678; (d) Ren, L.; Cong, H. Visible-Light-Driven Decarboxylative Alkylation of C-H Bond Catalyzed by Dye-Sensitized Semiconductor. Org. Lett. 2018, 20, 3225–3228; (e) Tang, F.; Feng, Y.-S.; Cheng, Z.-F.; Zhang, Q.; Xu, H.-J. Intermolecular 1,2-Difunctionalization of Terminal Alkynes to Access Trisubstituted Alkenes via Copper/Photoredox Dual Catalysis. Org. Lett. 2023, 25, 3916–3921; (f) Yao, S.; Zhang, K.; Zhou, Q.-Q.; Zhao, Y.; Shi, D.-Q.; Xiao, W.-J. Photoredox-Promoted Alkyl Radical Addition/Semipinacol Rearrangement Sequences of Alkenylcyclobutanols: Rapid Access to Cyclic Ketones. Chem. Commun. 2018, 54, 8096–8099; (g) Zhao, Y.; Chen, J.-R.; Xiao, W.-J. Visible-Light Photocatalytic Decarboxylative Alkyl Radical Addition Cascade for Synthesis of Benzazepine Derivatives. Org. Lett. 2018, 20, 224-227.
- 7(a) Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y. Photocatalytic Decarboxylative Alkylations Mediated by Triphenylphosphine and Sodium Iodide. Science 2019, 363, 1429–1434; (b) Li, Q.; Zhu, Z.-Q.; Zhang, W.-Y.; Le, Z.-G.; Xie, Z.-B. Visible-Light-Induced Decarboxylative Cascade Cyclization of Acryloylbenzamides with N-hydroxyphthalimide Esters via EDA Complexes. Org. Biomol. Chem. 2024, 22, 965–969; (c) Liu, X.-J.; Zhou, S.-Y.; Xiao, Y.; Sun, Q.; Lu, X.; Li, Y.; Li, J.-H. Photocatalytic Decarboxylative [3 + 2] and [4 + 2] Annulation of Enynals and γ,σ-Unsaturated N-(Acyloxy)phthalimides by NaI/PPh3 Catalysis. Org. Lett. 2021, 23, 7839–7844; (d) Panda, S. P.; Dash, R.; Hota, S. K.; Murarka, S. Photodecarboxylative Radical Cascade Involving N-(Acyloxy)phthalimides for the Synthesis of Pyrazolones. Org. Lett. 2024, 26, 3667–3672; (e) Panda, S. P.; Hota, S. K.; Dash, R.; Roy, L.; Murarka, S. Photodecarboxylative C-H Alkylation of Azauracils with N-(Acyloxy)phthalimides. Org. Lett. 2023, 25, 3739–3744; (f) Su, Z.; Zhang, J.; Lin, H.-W.; Liao, H. Synthesis of 1,4-Dicarbonyl Compounds via Visible-Light-Induced Decarboxylative Radical Cascade Reactions. Adv. Synth. Catal. 2023, 365, 3444–3449; (g) Wadekar, K.; Aswale, S.; Yatham, V. R. PPh3/NaI Driven Photocatalytic Decarboxylative Radical Cascade Alkylarylation Reaction of 2-Isocyanobiaryls. RSC Adv. 2020, 10, 16510–16514; (h) Wang, Y.-T.; Fu, M.-C.; Zhao, B.; Shang, R.; Fu, Y. Photocatalytic Decarboxylative Alkenylation of α-Amino and α-Hydroxy Acid-Derived Redox Active Esters by NaI/PPh3 Catalysis. Chem. Commun. 2020, 56, 2495–2498.
- 8(a) Abdel-Halim, O. B.; Morikawa, T.; Ando, S.; Matsuda, H.; Yoshikawa, M. New Crinine-Type Alkaloids with Inhibitory Effect on Induction of Inducible Nitric Oxide Synthase from Crinum yemense. J. Nat. Prod. 2004, 67, 1119–1124; (b) Fan, H.; Peng, J.; Hamann, M. T.; Hu, J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108, 264–287; (c) Hu, J.-F.; Fan, H.; Xiong, J.; Wu, S.-B. Discorhabdins and Pyrroloiminoquinone-Related Alkaloids. Chem. Rev. 2011, 111, 5465–5491; (d) Naidu, K. M.; Nagesh, H. N.; Singh, M.; Sriram, D.; Yogeeswari, P.; Gowri Chandra Sekhar, K. V. Novel Amide and Sulphonamide Derivatives of 6-(Piperazin-1-yl)phenanthridine as Potent Mycobacterium Tuberculosis H37Rv Inhibitors. Eur. J. Med. Chem. 2015, 92, 415–426; (e) Zhang, Q.; Lei, H.; Zhou, C.-Y.; Wang, C. Construction of N-Polyheterocycles by N-Heterocyclic Carbene Catalysis via a Regioselective Intramolecular Radical Addition/Cyclization Cascade. Org. Lett. 2022, 24, 4615–4619.
- 9(a) Karthik, S.; Ajantha, J.; Easwaramoorthi, S.; Gandhi, T. Pyrenoimidazole-Fused Phenanthridine Derivatives with Intense Red Excimer Fluorescence in the Solid State. New J. Chem. 2020, 44, 9530–9539; (b) Lasák, P.; Motyka, K.; Kryštof, V.; Stýskala, J. Synthesis, Bacteriostatic and Anticancer Activity of Novel Phenanthridines Structurally Similar to Benzo[c]phenanthridine Alkaloids. Molecules 2018, 23, 2155; (c) Marten, I.; Podlech, J. Synthesis of Helical Indolophenanthridines Showing Aggregation-Induced Emission. Org. Lett. 2024, 26, 1148–1153; (d) Thirupataiah, B.; Reddy, G. S.; Ghule, S. S.; Kumar, J. S.; Mounika, G.; Hossain, K. A.; Mudgal, J.; Mathew, J. E.; Shenoy, G. G.; Parsa, K. V. L.; Pal, M. Synthesis of 11,12-Dihydro Benzo[c]phenanthridines via a Pd-Catalyzed Unusual Construction of Isocoumarin Ring/FeCl3-Mediated Intramolecular Arene-Allyl Cyclization: First Identification of a Benzo[c]phenanthridine Based PDE4 Inhibitor. Bioorg. Chem. 2020, 97, 103691; (e) Umamahesh, B.; Sathiskumar, U.; Augustine, G.; Easwaramoorthi, S.; Ayyadurai, N.; Sathiyanarayanan, K. I. Photophysical Studies of Donor, Acceptor Substituted Tetrahydrodibenzo[a,i]phenanthridines. Dyes Pigm. 2016, 134, 409–418; (f) Wan, M.; Zhang, L.; Chen, Y.; Li, Q.; Fan, W.; Xue, Q.; Yan, F.; Song, W. Synthesis and Anticancer Activity Evaluation of Novel Phenanthridine Derivatives. Front. Oncol. 2019, 9, 274; (g) Wang, Y.-T.; Long, X.-Y.; Ding, X.; Fan, S.-R.; Cai, J.-Y.; Yang, B.-J.; Zhang, X.-F.; Luo, R.-h.; Yang, L.; Ruan, T.; Ren, J.; Jing, C.-X.; Zheng, Y.-T.; Hao, X.-J.; Chen, D.-Z. Novel Nucleocapsid Protein-Targeting Phenanthridine Inhibitors of SARS-CoV-2. Eur. J. Med. Chem. 2022, 227, 113966; (h) Yuvaraj, A. R.; Renjith, A.; Kumar, S. Novel Electron-Deficient Phenanthridine Based Discotic Liquid Crystals. J. Mol. Liq. 2018, 272, 583–589.
- 10(a) Aravindan, N.; Jeganmohan, M. One-Pot Synthesis of Benzo[c]phenanthridine Alkaloids from 7-Azabenzonorbornadienes and Aryl Nitrones. Org. Lett. 2023, 25, 3853–3858; (b) Dey, A.; Kumar, V.; Chatterjee, R.; Behera, A.; Maurya, R. K.; Burra, A. G.; Kumar, S.; Khatravath, M.; Dandella, R. Recent Advancements in Synthesis of Phenanthridines via 2-Isocyanobiphenyls. Asian J. Org. Chem. 2023, 12, e202300369; (c) Jiang, P.; Shan, Z.; Chen, S.; Wang, Q.; Jiang, S.; Zheng, H.; Deng, G.-J. Metal-Free Synthesis of Benzo[a]phenanthridines from Aromatic Aldehydes, Cyclohexanones, and Aromatic Amines. Chin. J. Chem. 2022, 40, 365–370; (d) Li, J.; Wang, S.; Zhao, J.; Li, P. Visible Light-Promoted Radical-Mediated Ring-Opening/Cyclization of Vinyl Benzotriazoles: An Alternative Approach to Phenanthridines. Org. Lett. 2022, 24, 5977–5981; (e) Nozawa-Kumada, K.; Matsuzawa, Y.; Hayashi, M.; Kobayashi, T.; Shigeno, M.; Yada, A.; Kondo, Y. Copper-Catalyzed Aerobic Benzylic C(sp3)-H Oxidation of Unprotected Aniline Derivatives for the Synthesis of Phenanthridines. Adv. Synth. Catal. 2024, 366, 2241–2245; (f) Okamura, H.; Iida, M.; Kaneyama, Y.; Nagatsugi, F. o-Nitrobenzyl Oxime Ethers Enable Photoinduced Cyclization Reaction to Provide Phenanthridines under Aqueous Conditions. Org. Lett. 2023, 25, 466–470; (g) Talukdar, V.; Vijayan, A.; Kumar Katari, N.; Radhakrishnan, K. V.; Das, P. Recent Trends in the Synthesis and Mechanistic Implications of Phenanthridines. Adv. Synth. Catal. 2021, 363, 1202–1245.
- 11(a) Bao, H.; Zheng, L.; Liu, Q.; Han, M.; Li, Y.; Bao, M.; Li, Y.; Yan, P.; Liu, Y. A Photocatalytic Traceless C-N Bond Formation/Cleavage Strategy Enabling the Use of (α-Chiral) Alkyl Aldehydes as Deoxygenative (Chiral) Alkyl Radical Equivalents. Org. Chem. Front. 2023, 10, 5551–5558; (b) Chen, J.-Y.; Wu, H.-Y.; Song, H.-Y.; Li, H.-X.; Jiang, J.; Yang, T.-B.; He, W.-M. Visible-Light-Induced Annulation of Iodonium Ylides and 2-Isocyanobiaryls to Access 6-Arylated Phenanthridines. J. Org. Chem. 2023, 88, 8360–8368; (c) Doraghi, F.; Amini, A.; Ghanbarlou, M.; Larijani, B.; Mahdavi, M. Metal-Free 2-Isocyanobiaryl- Based Cyclization Reactions: Phenanthridine Framework Synthesis. Mol. Divers. 2024, 28, 419–435; (d) Li, Q.; Zhou, C.-Y.; Wang, C. Metal-Free Generation of γ-Cyanoalkyl Radicals by N-Heterocyclic Carbene Catalysis: Assembly of 6-Cyanoalkyl Phenanthridines. Org. Lett. 2022, 24, 9243–9247; (e) Wu, X.; Chen, P.; Gan, M.; Ji, X.; Deng, G.-J.; Huang, H. Redox-Neutral Cyclization of 2-Isocyanobiaryls through Photoredox/PPh3 Dual Catalysis. Org. Lett. 2023, 25, 9186–9190; (f) Zhang, Y.; Jin, Y.; Wang, L.; Zhang, Q.; Meng, C.; Duan, C. Selective C(sp3)-H Activation of Simple Alkanes: Visible Light-Induced Metal-Free Synthesis of Phenanthridines with H2O2 as a Sustainable Oxidant. Green Chem. 2021, 23, 6926–6930; (g) Zhou, N.; Wang, L.; Zhao, F.; Gao, X.; Zhao, X.; Zhang, M. NHC-Catalyzed Tandem Reaction: A Strategy for the Synthesis of 2-Pyrrolidinone-Functionalized Phenanthridines. J. Org. Chem. 2023, 88, 16556–16565.
- 12(a) Chen, F.; Quan, L.-X.; Zhou, A.; Ji, C.; Li, Y.; Zhu, X.; Mao, L.-L.; Wan, J.-P. Tandem Sulfonylation/Cyclization of Vinyl Azides with Aryldiazonium Tetrafluoroborates and SO2. Eur. J. Org. Chem. 2023, 26, e202201269; (b) Devi, L.; Pokhriyal, A.; Shekhar, S.; Kant, R.; Mukherjee, S.; Rastogi, N. Organo-photocatalytic Synthesis of 6-β-Disubstituted Phenanthridines from α-Diazo-β-Keto Compounds and Vinyl Azides. Asian J. Org. Chem. 2021, 10, 3328–3333; (c) Li, G.; Kong, X.; Liang, Q.; Lin, L.; Yu, K.; Xu, B.; Chen, Q. Metal-Free Electrochemical Coupling of Vinyl Azides: Synthesis of Phenanthridines and β-Ketosulfones. Eur. J. Org. Chem. 2020, 2020, 6135–6145; (d) Liang, Q.; Lin, L.; Li, G.; Kong, X.; Xu, B. Synthesis of Phenanthridine and Quinoxaline Derivatives via Copper-Catalyzed Radical Cyanoalkylation of Cyclobutanone Oxime Esters and Vinyl Azides. Chin. J. Chem. 2021, 39, 1948–1952; (e) Liu, L.; Zhang, Q.; Wang, C. Redox- Neutral Generation of Iminyl Radicals by N-Heterocyclic Carbene Catalysis: Rapid Access to Phenanthridines from Vinyl Azides. Org. Lett. 2022, 24, 5913–5917; (f) Sahu, T. K.; Vishwakarma, A.; Kumar, V.; Khan, R.; Khan, T. Thermal vs. Visible-Light Photoredox-Catalyzed Cascade Radical Cyclization Involving SO2 Fixation to Access 6-Alkylsulfonylmethyl Phenanthridines. Asian J. Org. Chem. 2024, 13, e202400022.
- 13(a) Chen, M.; Chen, J.-Q.; Chen, Z.; Wu, J. Synthesis of Ester-Containing Phenanthridines via Photoredox-Catalyzed Radical Cascade Cyclization of N-arylacrylamides with Alkyloxalyl Chlorides. Org. Chem. Front. 2023, 10, 3995–4001; (b) Li, X.; Fang, X.; Zhuang, S.; Liu, P.; Sun, P. Photoredox Catalysis: Construction of Polyheterocycles via Alkoxycarbonylation/Addition/Cyclization Sequence. Org. Lett. 2017, 19, 3580–3583; (c) Liu, C.; Yan, G.; Wu, Z.; Yang, Y.; Yang, L.; Wang, J.; Wan, Z.; Wei, J.; Lu, J.; Wei, S.; Yi, D. Photocatalytic [2+2+m] Cyclization of 2-Cyanoaryl Acrylamides with 2-Bromocarbonyls Involving C(sp3)-H Functionalization. Adv. Synth. Catal. 2023, 365, 4513–4519; (d) Shang, J.-Q.; Wang, X.-X.; Xin, Y.; Li, Y.; Zhou, B.; Li, Y.-M. Decarboxylative Cascade Cyclization of α-Keto Acids with 2-Cyano-3-arylaniline-derived Acrylamides. Org. Biomol. Chem. 2019, 17, 9447–9455; (e) Wu, L.-J.; Yang, Y.; Song, R.-J.; Yu, J.-X.; Li, J.-H.; He, D.-L. An Access to 1,3-Azasiline-Fused Quinolinones via Oxidative Heteroannulation Involving Silyl C(sp3)-H Functionalization. Chem. Commun. 2018, 54, 1367–1370; (f) Xia, W.-J.; Xin, Y.; Zhao, Z.-W.; Chen, X.; Wang, X.-X.; Li, Y.; Wang, G.; Li, Y.-M. Oxidative Cascade Cyclization of 2-Cyano-3-arylaniline Derived Acrylamides with Toluenes, Ethers, Aliphatic Alcohols or Simple Alkanes. Org. Chem. Front. 2020, 7, 1997–2002; (g) Yu, Y.; Yuan, W.; Huang, H.; Cai, Z.; Liu, P.; Sun, P. Visible-Light-Mediated Decarboxylative Alkylation Cascade Cyano Insertion/Cyclization of N-Arylacrylamides under Transition-Metal- Free Conditions. J. Org. Chem. 2018, 83, 1654–1660; (h) Zhu, S.-S.; Liu, J.-K.; Qin, L.-Z.; Wang, J.; Duan, X.; Yuan, X.; Qiu, J.-K.; Guo, K. Visible-Light-Promoted Cyanoalkylation/Cyclization Cascade Reaction to Assemble Polyheterocycles in Continuous Flow. J. Org. Chem. 2023, 88, 2057–2068.




