A General Electron Donor-Acceptor Photoactivation Using Oxime Esters Enabled Divergent Thioetherifications†
Maojian Lu
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorLiang-Liang Jiang
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorYue-Ming Xu
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorSanliang Li
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorCorresponding Author
Qing-Xiao Tong
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jian-Ji Zhong
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, Guangdong, 515063 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMaojian Lu
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorLiang-Liang Jiang
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorYue-Ming Xu
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorSanliang Li
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorCorresponding Author
Qing-Xiao Tong
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jian-Ji Zhong
College of Chemistry and Chemical Engineering, and Key (Guangdong-Hong Kong Joint) Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, Guangdong, 515063 China
E-mail: [email protected]; [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Abstract
Comprehensive Summary
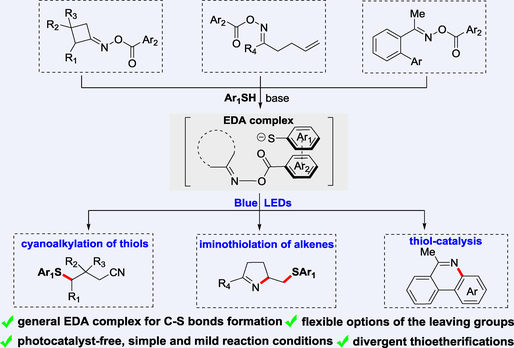
The EDA complex-mediated reactions involving oxime esters have been few studied. Herein, an EDA complex formed by thiophenolate anion and oxime ester is reported for photoinduced divergent synthesis of thioethers, depending on different types of oxime esters. Operational simplicity, mild reaction conditions, and flexible options of leaving group demonstrate the generality and synthetic utility of this approach. Such an approach can also enable an interesting thiol-catalysis for the synthesis of phenanthridines.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400564-sup-0001-supinfo.pdfPDF document, 8.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Liu, G.; Link, J.; Pei, T. Z.; Reilly, E. B.; Leitza, S.; Nguyen, B.; Marsh, K. C.; Okasinski, G. F.; Geldern, T. W.; Ormes, M.; Fowler, K.; Gallatin, M. Discovery of Novel p-Arylthio Cinnamides as Antagonists of Leukocyte Function–Associated Antigen-1/Intracellular Adhesion Molecule-1 Interaction. 1. Identification of an Additional Binding Pocket Based on an Anilino Diaryl Sulfide Lead. J. Med. Chem. 2000, 43, 4025–4040; (b) Feng, M.; Tang, B.; Liang, S. H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216; (c) Beletskaya, I. P.; Ananikov, V. P. Transition-Metal-Catalyzed C—S, C—Se, and C—Te Bond Formations via Cross-Coupling and Atom–Economic Addition Reactions. Achievements and Challenges. Chem. Rev. 2022, 122, 16110–16293; (d) Wu, Z.; Pratt, D. A. Radical approaches to C—S bonds. Nat. Rev. Chem. 2023, 7, 573–589.
- 2(a) Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T. S. A.; Liu, X. Recent Advances in C—S Bond Formation via C–H Bond Functionalization and Decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314; (b) Wimmer, A.; König, B. Photocatalytic Formation of Carbon–Sulfur Bonds. Beilstein J. Org. Chem. 2018, 14, 54–83; (c) Li, Y.; Huang, Z.; Mo, G.; Jiang, W.; Zheng, C.; Feng, P.; Ruan, Z. Direct Electrochemical Synthesis of Sulfur-Containing Triazolium Inner Salts. Chin. J. Chem. 2021, 39, 942–946; (d) Yang, Q.; Luo, H. Y.; Zhu, D.; Zhang, X. Y.; Ke, H.; Chen, Z. M., Chiral Lewis Base/Achiral Acid Co-Catalyzed Atroposelective Sulfenylation of Pyrrole Derivatives: Construction of C—N Axially Chiral Sulfides. Chin. J. Chem. 2024, 42, 2005—2009.
- 3(a) Arceo, E.; Jurberg, I. D.; Álvarez-Fernández, A.; Melchiorre, P. Photochemical Activity of a Key Donor–Acceptor Complex can Drive Stereoselective Catalytic α-Alkylation of Aldehydes. Nat. Chem. 2013, 5, 750–756;
(b) Kandukuri, S. R.; Bahamonde, A.; Chatterjee, I. Jurberg, I. D.; Escudero-Adán, E. C.; Melchiorre, P. X-Ray Characterization of an Electron Donor–Acceptor Complex that Drives the Photochemical Alkylation of Indoles. Angew. Chem. Int. Ed. 2015, 54, 1485–1489;
(c) Lima, C. G. S.; Lima, T. M.; Duarte, M. I.; Jurberg, D.; Paixão, M. W. Organic Synthesis Enabled by Light–Irradiation of EDA Complexes: Theoretical Background and Synthetic Applications. ACS Catal. 2016, 6, 1389–1407;
(d) Zhang, J.; Li, Y.; Xu, R.; Chen, Y. Donor–Acceptor Complex Enables Alkoxyl Radical Generation for Metal–Free C(sp3)—C(sp3) Cleavage and Allylation/Alkenylation. Angew. Chem., Int. Ed. 2017, 56, 12619–12623;
(e) Crisenza, G. E. M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor–Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476;
(f) Shen, J.; Li, J.; Chen, M.; Chen, Y. Photocatalyst–Free, Metal–Free, Visible– Light–Induced Thiolation/Pyridylation of Styrenes using an Electron Donor–Acceptor Complex as a Bifunctional Reagent. Org. Chem. Front. 2023, 10, 1166–1172;
(g) Jiang, Y. X.; Liao, L. L.; Gao, T. Y.; Xu, W. H.; Zhang, W.; Song, L.; Sun, G. Q.; Ye, J. H.; Lan, Y.; Yu, D. G. Visible–Light-Driven Synthesis of N-Heteroaromatic Carboxylic Acids by Thiolate-Catalysed Carboxylation of C(sp2) —H Bonds using CO2. Nat. Synth. 2024, 3, 394–405;
10.1038/s44160-023-00465-6 Google Scholar(h) Shen, J.; Li, J.; Chen, M.; Yue, X.; Shi, X. Photoinduced Radical Desulfurative C(sp3)—C(sp2) Coupling via Electron Donor–Acceptor Complexes. Org. Lett. 2024, 26, 1495–1500.
- 4(a) Chen, Z.; Xue, F.; Liu, T.; Wang, B.; Zhang, Y.; Jin, W.; Xia, Y.; Liu, C. Synthesis of β-Hydroxysulfides via Visible–Light–Driven and EDA Complex-Promoted Hydroxysulfenylation of Styrenes with Heterocyclic Thiols in EtOH under Photocatalyst–Free Conditions. Green Chem. 2022, 24, 3250–3256; (b) Tian, Y. M.; Hofmann, E.; Silva, W.; Pu, X.; Touraud, D.; Gschwind, R. M.; Kunz, W.; König, B. Enforced Electronic-Donor–Acceptor Complex Formation in Water for Photochemical Cross–Coupling. Angew. Chem. Int. Ed. 2023, 62, e202218775; (c) Piedra, H. F.; Plaza, M. Photochemical Halogen–Bonding Assisted Generation of Vinyl and Sulfur–Centered Radicals: Stereoselective Catalyst–Free C(sp2)—S Bond forming Reactions. Chem. Sci. 2023, 14, 650–657; (d) Hou, J. Y.; Zhang, L.; He, S. Y.; Ye, M. L.; Chen, J.; Huang, T. L.; Lv, G. H.; Hai, L.; Yang, Z. Z.; Wu, Y. Visible–Light–Induced Electron Donor–Acceptor (EDA) Complex–Initiated Synthesis of Non-Anomeric S-Aryl Glycosides. Org. Chem. Front. 2023, 10, 6200–6204; (e) Donnier-Valentin, L.; Kassamba, S.; Legros, J.; Fressigné, C.; Vuluga, D.; Brown, R. C. D.; Linclau, B.; De Paolis, M. Photoinduced Formation of Cubyl Aryl Thioethers and Synthesis of Monocubyl Analogue of Dapsone. Org. Lett. 2023, 25, 8580–8584.
- 5 Liu, B.; Lim, C. H.; Miyake, G. M. Visible–Light–Promoted C—S Cross–Coupling via Intermolecular Charge Transfer. J. Am. Chem. Soc. 2017, 139, 13616–13619.
- 6 Yang, M.; Cao, T.; Xu, T.; Liao, S. Visible–Light–Induced Deaminative Thioesterification of Amino Acid Derived Katritzky Salts via Electron Donor–Acceptor Complex Formation. Org. Lett. 2019, 21, 8673–8678.
- 7 Jin, Y.; Yang H.; Fu, H. An N-(Acetoxy)phthalimide Motif as a Visible–Light Pro-photosensitizer in Photoredox Decarboxylative Arylthiation. Chem. Commun. 2016, 52, 12909–12912.
- 8 Cai, Y. P.; Nie, F. Y.; Song, Q. H. Visible–Light–Mediated Alkylation of Thiophenols via Electron Donor–Acceptor Complexes Formed between Two Reactants. J. Org. Chem. 2021, 86, 12419–12426.
- 9(a) Uchikura, T.; Hara, Y.; Tsubono, K.; Akiyama, T. Visible–Light–Driven C—S Bond Formation Based on Electron Donor–Acceptor Excitation and Hydrogen Atom Transfer Combined System. ACS Org. Inorg. Au 2021, 1, 23–28; (b) Li, T.; Liang, K.; Tang, J.; Ding, Y.; Tong, X.; Xia, C. A Photoexcited Halogen-Bonded EDA Complex of the Thiophenolate anion with Iodobenzene for C(sp3)—H Activation and Thiolation. Chem. Sci. 2021, 12, 15655–15661; (c) Cabrera-Afonso, M. J.; Granados, A.; Molander, G. A. Sustainable Thioetherification via Electron Donor–Acceptor Photoactivation Using Thianthrenium Salts. Angew. Chem. Int. Ed. 2022, 61, e202202706.
- 10(a) Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Visible–Light–Promoted Iminyl-Radical Formation from Acyl Oximes: A Unified Approach to Pyridines, Quinolines, and Phenanthridines. Angew. Chem. Int. Ed. 2015, 54, 4055–4059; (b) Yu, X. Y.; Chen, J. R.; Wang, P. Z.; Yang, M. N.; Liang, D.; Xiao, W. J. A Visible–Light–Driven Iminyl Radical–Mediated C—C Single Bond Cleavage/Radical Addition Cascade of Oxime Esters. Angew. Chem. Int. Ed. 2018, 57, 738–743; (c) Morcillo, S. P.; Dauncey, E. M.; Kim, J. H.; Douglas, J. J.; Sheikh, N. S.; Leonori, D. Photoinduced Remote Functionalization of Amides and Amines Using Electrophilic Nitrogen Radicals. Angew. Chem. Int. Ed. 2018, 57, 12945–12949; (d) Yang, L.; Gao, P.; Duan, X. H.; Gu, Y. R.; Guo, L. N. Direct C—H Cyanoalkylation of Quinoxalin-2(1H)-ones via Radical C—C Bond Cleavage. Org. Lett. 2018, 20, 1034–1037; (e) Zhang, J.; Li, X.; Xie, W.; Ye, S.; Wu, J. Photoredox–Catalyzed Sulfonylation of O-Acyl Oximes via Iminyl Radicals with the Insertion of Sulfur Dioxide. Org. Lett. 2019, 21, 4950–4954; (f) Zhang, J. J.; Duan, X. H.; Wu, Y.; Yang, J. C.; Guo, L. N. Transition–Metal Free C—C Bond Cleavage/Borylation of Cycloketone Oxime Esters. Chem. Sci. 2019, 10, 161–166; (g) Anand, D.; He, Y.; Li, L.; Zhou, L. A Photocatalytic sp3 C—S, C—Se and C—B Bond Formation through C—C Bond Cleavage of Cycloketone Oxime Esters. Org. Biomol. Chem. 2019, 17, 533–540; (h) Song, C.; Shen, X.; Yu, F.; He, Y.; Yu, S. Generation and Application of Iminyl Radicals from Oxime Derivatives Enabled by Visible Light Photoredox Catalysis. Chin. J. Org. Chem. 2020, 40, 3748–3759; (i) Chen, J.; Liang, Y. J.; Wang, P. Z.; Li, G. Q.; Zhang, B.; Qian, H.; Huan, X. D.; Guan, W.; Xiao, W. J.; Chen, J. R. Photoinduced Copper-Catalyzed Asymmetric C—O Cross–Coupling. J. Am. Chem. Soc. 2021, 143, 13382–13392; (j) Liang, Q.; Lin, L.; Li, G.; Kong, X.; Xu, B. Synthesis of Phenanthridine and Quinoxaline Derivatives via Copper-Catalyzed Radical Cyanoalkylation of Cyclobutanone Oxime Esters and Vinyl Azides. Chin. J. Chem. 2021, 39, 1948–1952; (k) Zhang, J.; Tang, J.; Chen, Z.; Wu, X. F. Elemental Sulfur and Dimethyl Sulfoxide–Promoted Oxidative Cyclization of Trifluoroacetimidohydrazides with Methylhetarenes for the Synthesis of 3-Hetaryl-5-trifluoromethyl-1, 2,4-triazoles. Chin. J. Chem. 2021, 39, 3443–3447; (l) Shan, Q. C.; Zhao, Y.; Wang, S. T.; Liu, H. F.; Duan, X. H.; Guo, L. N. Nickel–Catalyzed Modular Four-Component 1,4-Alkylcarbonylation of 1,3-Enynes to Tetra-Substituted CF3–Allenyl Ketones. ACS Catal. 2024, 14, 2144–2150; (m) Bao, Y.; Song, Z. J.; Dai, J. L.; Yan, S.; Zhang, Y.; Wang, J. Y.; Li, G. Stereospecific Assembly of Trisubstituted Alkenes via Photoinduced Nitrogen-Centered Radical–Triggered C—C Bond Cleavage/Functionalization of Oxime Esters. Chin. J. Chem. 2024, 42, 1399–1406.
- 11 Davies, J.; Booth, S. G.; Essafi, S.; Dryfe, R. A. W.; Leonori, D. Visible–Light–Mediated Generation of Nitrogen–Centered Radicals: Metal–Free Hydroimination and Iminohydroxylation Cyclization Reactions. Angew. Chem. Int. Ed. 2015, 54, 14017–14021.
- 12 Li, Y.; Mao, R.; Wu, J. N-Radical Initiated Aminosulfonylation of Unactivated C(sp3)—H Bond through Insertion of Sulfur Dioxide. Org. Lett. 2017, 19, 4472–4475.
- 13 Sun, J.; He, Y.; An, X. D.; Zhang, X.; Yu, L.; Yu, S. Visible–Light–Induced Iminyl Radical Formation via Electron–Donor–Acceptor Complexes: a Photocatalyst–Free Approach to Phenanthridines and Quinolones. Org. Chem. Front. 2018, 5, 977–981.
- 14 Zheng, D.; Jana, K.; Alasmary, F. A.; Daniliuc, C. G.; Studer, A. Transition–Metal–Free Intramolecular Radical Aminoboration of Unactivated Alkenes. Org. Lett. 2021, 23, 7688–7692.
- 15 Zhang, D. L.; Le, Z. G.; Li, Q.; Xie, Z. B.; Yang, W. W.; Zhu, Z. Q. Visible–Light–Driven EDA Complex-Promoted Cascade Cyclization to Construct 4-Cyanoalkyl Isoquinoline-1,3-Diones. Chem. Commun. 2024, 60, 2958–2961.
- 16(a) Xiao, Q.; Zhang, H.; Li, J. H.; Jian, J. X.; Tong, Q. X.; Zhong, J. J. Directing–Group–Assisted Markovnikov–Selective Hydrothiolation of Styrenes with Thiols by Photoredox/Cobalt Catalysis. Org. Lett. 2021, 23, 3604–3609; (b) Shi, J.; Gao, X. W.; Tong, Q. X.; Zhong, J. J. Light–Promoted and Tertiary–Amine–Assisted Hydroxysulfenylation of Alkenes: Selective and Direct One–Pot Synthesis of β-Hydroxysulfides. J. Org. Chem. 2021, 86, 12922–12931; (c) Liang, R. B.; Zhu, C. M.; Song, P. Q.; Zhao, L. M.; Tong, Q. X.; Zhong, J. J. External Oxidant–Free and Selective Thiofunctionalization of Alkenes enabled by Photoredox–Neutral Catalysis. Org. Chem. Front. 2022, 9, 4536–4541; (d) Xiao, Q.; Tong, Q. X.; Zhong, J. J. Recent Advances in Visible–Light Photoredox Catalysis for the Thiol-Ene/Yne Reactions. Molecules 2022, 27, 619–641; (e) Zhu, C. M.; Liang, R. B.; Xiao, Y.; Zhou, W.; Tong, Q. X.; Zhong, J. J. Metal–Free and Site-Selective α-C—H Functionalization of Tetrahydrofuran enabled by the Photocatalytic Generation of Bromine Radicals. Green Chem. 2023, 25, 960–965; (f) Lu, M. Liang, R. B.; Zhu, C. M.; Tong, Q. X.; Zhong, J. J. Photoredox Synthesis of Thio-Functionalized Cyclic Ethers Using N-Sulfenyl Phthalimides as a Thiyl-Radical Precursor. Chin. J. Chem. 2023, 41, 1823–1828; (g) Zhang, R. J.; Li, X. R.; Liang, R. B.; Xiao, Y.; Tong, Q. X.; Zhong, J. J.; Wu, L. Z. Thiyl Radical Trapped by Cobalt Catalysis: An Approach to Markovnikov Thiol–Ene Reaction. Org. Lett. 2024, 26, 591–596; (h) Li, X. R.; Zhang, R. J.; Xiao, Y.; Tong, Q. X.; Zhong, J. J. N-Sulfenyl Phthalimide enabled Markovnikov Hydrothiolation of Unactivated Alkenes via Ligand Promoted Cobalt Catalysis. Org. Chem. Front. 2024, 11, 646–653; (i) Xiao, Q.; Zhong, J. J. The Radical Chemistry of N-Sulfenyl Phthalimides/Succinimides for C—S bonds formation. Tetrahedron Lett. 2024, 144, 155153.




