Understanding the Temperature Effect on Carbon-Carbon Coupling during CO2 and CO Electroreduction in Zero-Gap Electrolyzers†
Mengjiao Zhuansun
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorXuan Wang
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorWenzhi Teng
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Yuhang Wang
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]Search for more papers by this authorMengjiao Zhuansun
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorXuan Wang
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorWenzhi Teng
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Yuhang Wang
Institute of Functional Nano & Soft Materials (FUNSOM), Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
Jiangsu Key Laboratory for Advanced Negative Carbon Technologies, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
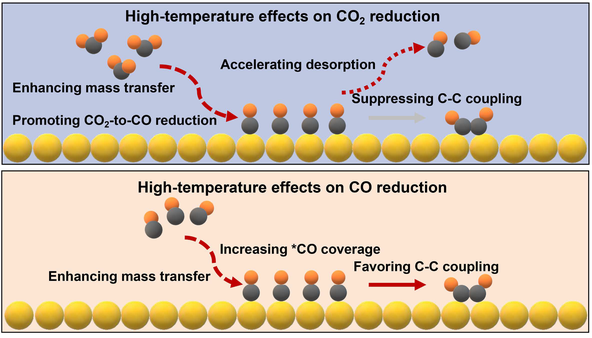
Cu-catalyzed electrochemical CO2 reduction reaction (CO2RR) and CO reduction reaction (CORR) are of great interest due to their potential to produce carbon-neutral and value-added multicarbon (C2+) chemicals. In practice, CO2RR and CORR are typically operated at industrially relevant current densities, making the process exothermal. Although the increased operation temperature is known to affect the performance of CO2RR and CORR, the relationship between temperatures and kinetic parameters was not clearly elaborated, particularly in zero-gap reactors. In this study, we detail the effect of the temperature on Cu-catalyzed CO2RR and CORR. Our electrochemical and operando spectroscopic studies show that high temperatures increase the activity of CO2RR to CO and CORR to C2H4 by enhancing the mass transfer of CO2 and CO. As the rates of these two processes are highly influenced by reactant diffusion, elevating the operating temperature results in high local CO2 and CO availability to accelerate product formation. Consequently, the *CO coverage in both cases increases at higher temperatures. However, under CO2RR conditions, *CO desorption is more favorable than carbon-carbon (C—C) coupling thermodynamically at high temperatures, causing the reduction in the Faradaic efficiency (FE) of C2H4. In CORR, the high-temperature-augmented CO diffusion overcomes the unfavorable adsorption thermodynamics, increasing the probability of C—C coupling.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400454-sup-0001-supinfo.pdfPDF document, 3.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Gao, D.; Arán-Ais, R. M.; Jeon, H. S.; Roldan Cuenya, B. Rational Catalyst and Electrolyte Design for CO2 Electroreduction towards Multicarbon Products. Nat. Catal. 2019, 2, 198–210.
- 2 Kintisch, E. After Paris: The Rocky Road Ahead. Science 2015, 350, 1018–1019.
- 3 Meys, R.; Kätelhön, A.; Bachmann, M.; Winter, B.; Zibunas, C.; Suh, S.; Bardow, A. Achieving Net-Zero Greenhouse Gas Emission Plastics by a Circular Carbon Economy. Science 2021, 374, 71–76.
- 4 Whipple, D. T.; Kenis, P. J. A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458.
- 5 Liu, M.; Wang, Q.; Luo, T.; Herran, M.; Cao, X.; Liao, W.; Zhu, L.; Li, H.; Stefancu, A.; Lu, Y.-R.; Chan, T.-S.; Pensa, E.; Ma, C.; Zhang, S.; Xiao, R.; Cortés, E. Potential Alignment in Tandem Catalysts Enhances CO2- to-C2H4 Conversion Efficiencies. J. Am. Chem. Soc. 2024, 146, 468–475.
- 6 Chen, Q.; Wang, X.; Zhou, Y.; Tan, Y.; Li, H.; Fu, J.; Liu, M. Electrocatalytic CO2 Reduction to C2+ Products in Flow Cells. Adv. Mater. 2024, 36, 2303902.
- 7 Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 Conversion: From Fundamentals to Value-Added Products. Chem. Soc. Rev. 2021, 50, 4993–5061.
- 8 Nitopi, S.; Bertheussen, E.; Scott, S. B.; Liu, X.; Engstfeld, A. K.; Horch, S.; Seger, B.; Stephens, I. E. L.; Chan, K.; Hahn, C.; Nørskov, J. K.; Jaramillo, T. F.; Chorkendorff, I. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672.
- 9 Jouny, M.; Luc, W.; Jiao, F. High-Rate Electroreduction of Carbon Monoxide to Multi-Carbon Products. Nat. Catal. 2018, 1, 748–755.
- 10 Jouny, M.; Hutchings, G. S.; Jiao, F. Carbon Monoxide Electroreduction as an Emerging Platform for Carbon Utilization. Nat. Catal. 2019, 2, 1062–1070.
- 11 Li, C. W.; Ciston, J.; Kanan, M. W. Electroreduction of Carbon Monoxide to Liquid Fuel on Oxide-Derived Nanocrystalline Copper. Nature 2014, 508, 504–507.
- 12 Yang, B.; Liu, K.; Li, H.; Liu, C.; Fu, J.; Li, H.; Huang, J. E.; Ou, P.; Alkayyali, T.; Cai, C.; Duan, Y.; Liu, H.; An, P.; Zhang, N.; Li, W.; Qiu, X.; Jia, C.; Hu, J.; Chai, L.; Lin, Z.; Gao, Y.; Miyauchi, M.; Cortés, E.; Maier, S. A.; Liu, M. Accelerating CO2 Electroreduction to Multicarbon Products via Synergistic Electric–Thermal Field on Copper Nanoneedles. J. Am. Chem. Soc. 2022, 144, 3039–3049.
- 13 Krause, R.; Reinisch, D.; Reller, C.; Eckert, H.; Hartmann, D.; Taroata, D.; Wiesner-Fleischer, K.; Bulan, A.; Lueken, A.; Schmid, G. Industrial Application Aspects of the Electrochemical Reduction of CO2 to CO in Aqueous Electrolyte. Chem. Ing. Tech. 2020, 92, 53–61.
- 14 Lobaccaro, P.; Singh, M. R.; Clark, E. L.; Kwon, Y.; Bell, A. T.; Ager, J. W. Effects of Temperature and Gas–Liquid Mass Transfer on the Operation of Small Electrochemical Cells for the Quantitative Evaluation of CO2 Reduction Electrocatalysts. Phys. Chem. Chem. Phys. 2016, 18, 26777–26785.
- 15 Iglesias Van Montfort, H.-P.; Burdyny, T. Mapping Spatial and Temporal Electrochemical Activity of Water and CO2 Electrolysis on Gas-Diffusion Electrodes Using Infrared Thermography. ACS Energy Lett. 2022, 7, 2410–2419.
- 16 Ahn, S. T.; Abu-Baker, I.; Palmore, G. T. R. Electroreduction of CO2 on Polycrystalline Copper: Effect of Temperature on Product Selectivity. Catal. Today 2017, 288, 24–29.
- 17 Vos, R. E.; Kolmeijer, K. E.; Jacobs, T. S.; Van Der Stam, W.; Weckhuysen, B. M.; Koper, M. T. M. How Temperature Affects the Selectivity of the Electrochemical CO2 Reduction on Copper. ACS Catal. 2023, 13, 8080–8091.
- 18 Proietto, F.; Rinicella, R.; Galia, A.; Scialdone, O. Electrochemical Conversion of CO2 to Formic Acid Using a Sn Based Cathode: Combined Effect of Temperature and Pressure. J. CO2 Util. 2023, 67, 102338.
- 19 Piontek, S.; Andronescu, C.; Zaichenko, A.; Konkena, B.; Junge Puring, K.; Marler, B.; Antoni, H.; Sinev, I.; Muhler, M.; Mollenhauer, D.; Roldan Cuenya, B.; Schuhmann, W.; Apfel, U.-P. Influence of the Fe: Ni Ratio and Reaction Temperature on the Efficiency of (FexNi1–x)9S8 Electrocatalysts Applied in the Hydrogen Evolution Reaction. ACS Catal. 2018, 8, 987–996.
- 20 Li, T.; Kang, S.; Zhang, X.; Fu, X.; Feng, S.; Hu, Z.; Zhu, D.; Lu, W. Improved Hydrogen Evolution at High Temperature Using an Electro-Thermal Method. J. Phys. D: Appl. Phys. 2020, 53, 185302.
- 21 Shafaque, H. W.; Lee, J. K.; Krause, K.; Lee, C.; Fahy, K. F.; Shrestha, P.; Balakrishnan, M.; Bazylak, A. Temperature Enhances the Ohmic and Mass Transport Behaviour in Membrane Electrode Assembly Carbon Dioxide Electrolyzers. Energy Convers. Manage. 2021, 243, 114302.
- 22 Tamimi, A.; Rinker, E. B.; Sandall, O. C. Diffusion Coefficients for Hydrogen Sulfide, Carbon Dioxide, and Nitrous Oxide in Water over the Temperature Range 293-368 K. J. Chem. Eng. Data 1994, 39, 330–332.
- 23 Lu, W.; Guo, H.; Chou, I. M.; Burruss, R. C.; Li, L. Determination of Diffusion Coefficients of Carbon Dioxide in Water between 268 and 473 K in a High-Pressure Capillary Optical Cell with in situ Raman Spectroscopic Measurements. Geochim. Cosmochim. Acta 2013, 115, 183–204.
- 24 Proietto, F.; Rinicella, R.; Galia, A.; Scialdone, O. Electrochemical Conversion of CO2 to Formic Acid Using a Sn Based Cathode: Combined Effect of Temperature and Pressure. J. CO2 Util. 2023, 67, 102338.
- 25 Zong, Y.; Chakthranont, P.; Suntivich, J. Temperature Effect of CO2 Reduction Electrocatalysis on Copper: Potential Dependency of Activation Energy. J. Electrochem. Energy Convers. Storage 2020, 17, 041007.
- 26 Zhao, K.; Quan, X. Carbon-Based Materials for Electrochemical Reduction of CO2 to C2+ Oxygenates: Recent Progress and Remaining Challenges. ACS Catal. 2021, 11, 2076–2097.
- 27 Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 Conversion: From Fundamentals to Value-Added Products. Chem. Soc. Rev. 2021, 50, 4993–5061.
- 28 Ji, Y.; Guan, A.; Zheng, G. Copper-Based Catalysts for Electrochemical Carbon Monoxide Reduction. Cell Rep. Phys. Sci. 2022, 3, 101072.
- 29 Wang, Y.; Liu, J.; Zheng, G. Designing Copper-Based Catalysts for Efficient Carbon Dioxide Electroreduction. Adv. Mater. 2021, 33, 2005798.
- 30 Li, K.; Zou, S.; Zhang, J.; Huang, Y.; He, L.; Feng, X. Superhydrophobicity-Enabled Efficient Electrocatalytic CO2 Reduction at a High Temperature. ACS Catal. 2023, 13, 9346–9351.
- 31 Keil, F. J. Kinetics of Chemical Reactions. Decoding Complexity. By Guy Marin and Gregory S. Yablonsky. Angew. Chem. Int. Ed. 2012, 51, 7080–7081.
- 32 Vos, R. E.; Koper, M. T. M. The Effect of Temperature on the Cation-Promoted Electrochemical CO2 Reduction on Gold. ChemElectroChem 2022, 9, e202200239.
- 33 Abouzari-Lotf, E.; Jacob, M. V.; Ghassemi, H.; Zakeri, M.; Nasef, M. M.; Abdolahi, Y.; Abbasi, A.; Ahmad, A. Highly Conductive Anion Exchange Membranes Based on Polymer Networks Containing Imidazolium Functionalised Side Chains. Sci. Rep. 2021, 11, 3764.
- 34 Weng, L.-C.; Bell, A. T.; Weber, A. Z. Towards Membrane-Electrode Assembly Systems for CO2 Reduction: A Modeling Study. Energy Environ. Sci. 2019, 12, 1950–1968.
- 35 Giron Rodriguez, C. A.; Kani, N. C.; Moss, A. B.; Joensen, B. O.; Garg, S.; Deng, W.; Wilson, T.; Varcoe, J. R.; Chorkendorff, I.; Seger, B. Insights into Zero-Gap CO2 Electrolysis at Elevated Temperatures. EES Catal. 2024, 10, 1039.
- 36 Moss, B.; Svane, K. L.; Nieto-Castro, D.; Rao, R. R.; Scott, S. B.; Tseng, C.; Sachs, M.; Pennathur, A.; Liang, C.; Oldham, L. I.; Mazzolini, E.; Jurado, L.; Sankar, G.; Parry, S.; Celorrio, V.; Dawlaty, J. M.; Rossmeisl, J.; Galán-Mascarós, J. R.; Stephens, I. E. L.; Durrant, J. R. Cooperative Effects Drive Water Oxidation Catalysis in Cobalt Electrocatalysts through the Destabilization of Intermediates. J. Am. Chem. Soc. 2024, 146, 8915–8927.
- 37 Zhan, C.; Dattila, F.; Rettenmaier, C.; Bergmann, A.; Kühl, S.; García-Muelas, R.; López, N.; Cuenya, B. R. Revealing the CO Coverage-Driven C–C Coupling Mechanism for Electrochemical CO2 Reduction on Cu 2 O Nanocubes via Operando Raman Spectroscopy. ACS Catal. 2021, 11, 7694–7701.
- 38 Gunathunge, C. M.; Li, X.; Li, J.; Hicks, R. P.; Ovalle, V. J.; Waegele, M. M. Spectroscopic Observation of Reversible Surface Reconstruction of Copper Electrodes under CO2 Reduction. J. Phys. Chem. C 2017, 121, 12337–12344.
- 39 Jiang, S.; Klingan, K.; Pasquini, C.; Dau, H. New Aspects of Operando Raman Spectroscopy Applied to Electrochemical CO2 Reduction on Cu Foams. J. Chem. Phys. 2019, 150, 041718.
- 40 Moradzaman, M.; Mul, G. Infrared Analysis of Interfacial Phenomena during Electrochemical Reduction of CO2 over Polycrystalline Copper Electrodes. ACS Catal. 2020, 10, 8049–8057.
- 41 Zhu, S.; Jiang, B.; Cai, W.-B.; Shao, M. Direct Observation on Reaction Intermediates and the Role of Bicarbonate Anions in CO2 Electrochemical Reduction Reaction on Cu Surfaces. J. Am. Chem. Soc. 2017, 139, 15664–15667.
- 42 Moradzaman, M.; Mul, G. In Situ Raman Study of Potential-Dependent Surface Adsorbed Carbonate, CO, OH, and C Species on Cu Electrodes During Electrochemical Reduction of CO2. ChemElectroChem 2021, 8, 1478–1485.
- 43 Chen, X.; Chen, J.; Alghoraibi, N. M.; Henckel, D. A.; Zhang, R.; Nwabara, U. O.; Madsen, K. E.; Kenis, P. J. A.; Zimmerman, S. C.; Gewirth, A. A. Electrochemical CO2-to-Ethylene Conversion on Polyamine-Incorporated Cu Electrodes. Nat. Catal. 2020, 4, 20–27.
- 44 Kong, X.; Zhao, J.; Ke, J.; Wang, C.; Li, S.; Si, R.; Liu, B.; Zeng, J.; Geng, Z. Understanding the Effect of *CO Coverage on C–C Coupling toward CO 2 Electroreduction. Nano Lett. 2022, 22, 3801–3808.
- 45 Zhao, Y.; Zu, X.; Chen, R.; Li, X.; Jiang, Y.; Wang, Z.; Wang, S.; Wu, Y.; Sun, Y.; Xie, Y. Industrial-Current-Density CO2 -to-C2+ Electroreduction by Anti-Swelling Anion-Exchange Ionomer-Modified Oxide-Derived Cu Nanosheets. J. Am. Chem. Soc. 2022, 144, 10446–10454.
- 46 Hou, J.; Chang, X.; Li, J.; Xu, B.; Lu, Q. Correlating CO Coverage and CO Electroreduction on Cu via High-Pressure in Situ Spectroscopic and Reactivity Investigations. J. Am. Chem. Soc. 2022, 144, 22202–22211.
- 47 Ren, D.; Fong, J.; Yeo, B. S. The Effects of Currents and Potentials on the Selectivities of Copper toward Carbon Dioxide Electroreduction. Nat. Commun. 2018, 9, 925.
- 48 Kim, C.; Bui, J. C.; Luo, X.; Cooper, J. K.; Kusoglu, A.; Weber, A. Z.; Bell, A. T. Tailored Catalyst Microenvironments for CO2 Electroreduction to Multicarbon Products on Copper Using Bilayer Ionomer Coatings. Nat. Energy 2021, 6, 1026–1034.




