Poly[2]catenane Gels Based on Sequential Assembly of Small Molecules†
Hanwei Zhang
Xi'an Rare Metal Materials Institute Co. Ltd., Xi'an, Shaanxi, 710016 China
Search for more papers by this authorJinsa Li
Key Laboratory of Materials Chemistry for Energy Conversion and Storage, Ministry of Education, Hubei Key Laboratory of Materials Chemistry and Service Failure, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, State Key Laboratory of Materials Processing and Die & Mould Technology, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, Hubei, 430074 China
Search for more papers by this authorZiqing Hu
Key Laboratory of Materials Chemistry for Energy Conversion and Storage, Ministry of Education, Hubei Key Laboratory of Materials Chemistry and Service Failure, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, State Key Laboratory of Materials Processing and Die & Mould Technology, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, Hubei, 430074 China
Search for more papers by this authorCorresponding Author
Xiaofan Ji
Key Laboratory of Materials Chemistry for Energy Conversion and Storage, Ministry of Education, Hubei Key Laboratory of Materials Chemistry and Service Failure, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, State Key Laboratory of Materials Processing and Die & Mould Technology, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, Hubei, 430074 China
E-mail: [email protected]Search for more papers by this authorHanwei Zhang
Xi'an Rare Metal Materials Institute Co. Ltd., Xi'an, Shaanxi, 710016 China
Search for more papers by this authorJinsa Li
Key Laboratory of Materials Chemistry for Energy Conversion and Storage, Ministry of Education, Hubei Key Laboratory of Materials Chemistry and Service Failure, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, State Key Laboratory of Materials Processing and Die & Mould Technology, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, Hubei, 430074 China
Search for more papers by this authorZiqing Hu
Key Laboratory of Materials Chemistry for Energy Conversion and Storage, Ministry of Education, Hubei Key Laboratory of Materials Chemistry and Service Failure, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, State Key Laboratory of Materials Processing and Die & Mould Technology, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, Hubei, 430074 China
Search for more papers by this authorCorresponding Author
Xiaofan Ji
Key Laboratory of Materials Chemistry for Energy Conversion and Storage, Ministry of Education, Hubei Key Laboratory of Materials Chemistry and Service Failure, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, State Key Laboratory of Materials Processing and Die & Mould Technology, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, Hubei, 430074 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
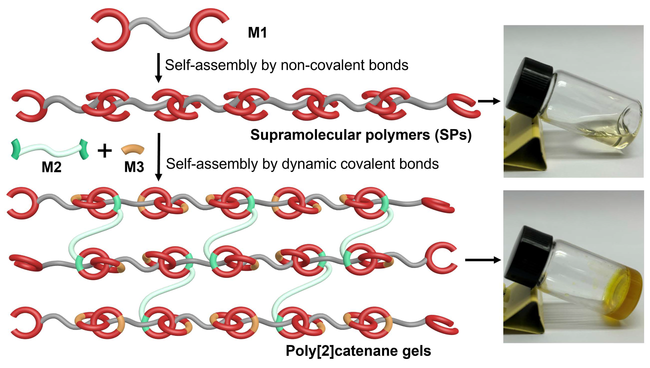
Polycatenane gels have attracted extensive attention due to their high degree of freedom and mobility. However, the syntheses of poly[2]catenane gels reported to date all rely on the polymer as the backbone. Herein, we prepared poly[2]catenane gels based on entirely sequential assembly of small molecules. Monomer M1 with two unclosed rings was first prepared, which self-assembled to form supramolecular polymers (SPs) via hydrogen bonding and π-π interactions. Upon adding small molecule monomers M2 and M3 with aldehyde groups, ring closing of SPs occurred because the amino groups in the SPs reacted with M2 to form imine bonds. In addition, M3, which had twice the number of aldehyde groups as M2, enabled SPs to ring-close, causing the proceeding of crosslinking process at the same time. Thus linear SPs were transformed into poly[2]catenane gel networks. Due to the presence of hydrogen bonds in the poly[2]catenane gel, the gel also possessed stimulus responsiveness and self-healing properties.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400435-sup-0001-supinfo.pdfPDF document, 1.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Mena-Hernando, S.; Pérez, E. M. Mechanically interlocked materials. Rotaxanes and catenanes beyond the small molecule. Chem. Soc. Rev. 2019, 48, 5016–5032.
- 2 Evans, N. H.; Beer, P. D. Progress in the synthesis and exploitation of catenanes since the Millennium. Chem. Soc. Rev. 2014, 43, 4658–4683.
- 3 Li, G.; Wang, L.; Wu, L.; Guo, Z.; Zhao, J.; Liu, Y.; Bai, R.; Yan, X. Woven Polymer Networks via the Topological Transformation of a [2]Catenane. J. Am. Chem. Soc. 2020, 142, 14343–14349.
- 4 Niu, Z.; Gibson, H. W. Polycatenanes. Chem. Rev. 2009, 109, 6024–6046.
- 5 Liu, G.; Rauscher, P. M.; Rawe, B. W.; Tranquilli, M. M.; Rowan, S. J. Polycatenanes: synthesis, characterization, and physical understanding. Chem. Soc. Rev. 2022, 51, 4928–4948.
- 6 Wang, J.; You, W.; Chen, L.; Xiao, D.; Xiao, X.; Shan, T.; Liu, Y.; Liu, M.; Li, G.; Yu, W.; Huang, F. Adaptive and Robust Vitrimers Fabricated by Synergy of Traditional and Supramolecular Polymers. Angew. Chem. Int. Ed. 2024, 63, e202405761.
- 7 Wang, L.; Cheng, L.; Li, G.; Liu, K.; Zhang, Z.; Li, P.; Dong, S.; Yu, W.; Huang, F.; Yan, X. A Self-Cross-Linking Supramolecular Polymer Network Enabled by Crown-Ether-Based Molecular Recognition. J. Am. Chem. Soc. 2020, 142, 2051–2058.
- 8 Wei, P.; Yan, X.; Cook, T. R.; Ji, X.; Stang, P. J.; Huang, F. Supramolecular Copolymer Constructed by Hierarchical Self-Assembly of Orthogonal Host–Guest, H-Bonding, and Coordination Interactions. ACS Macro Lett. 2016, 5, 671–675.
- 9 Olson, M. A.; Braunschweig, A. B.; Fang, L.; Ikeda, T.; Klajn, R.; Trabolsi, A.; Wesson, P. J.; Benítez, D.; Mirkin, C. A.; Grzybowski, B. A.; Stoddart, J. F. A Bistable Poly[2]catenane Forms Nanosuperstructures. Angew. Chem. Int. Ed. 2009, 48, 1792–1797.
- 10 Wang, J.; Ferguson, A. L. A Study of the Morphology, Dynamics, and Folding Pathways of Ring Polymers with Supramolecular Topological Constraints Using Molecular Simulation and Nonlinear Manifold Learning. Macromolecules 2018, 51, 598–616.
- 11 Okada, M.; Harada, A. Poly(polyrotaxane): Photoreactions of 9-Anthracene-Capped Polyrotaxane. Macromolecules 2003, 36, 9701–9703.
- 12 Higashi, T.; Morita, K.; Song, X.; Zhu, J.; Tamura, A.; Yui, N.; Motoyama, K.; Arima, H.; Li, J. One-pot synthesis of cyclodextrin-based radial poly[n]catenanes. Commun. Chem. 2019, 2, 78.
- 13 Li, A.; Tan, Z.; Hu, Y.; Lu, Z.; Yuan, J.; Li, X.; Xie, J.; Zhang, J.; Zhu, K. Precise Control of Radial Catenane Synthesis via Clipping and Pumping. J. Am. Chem. Soc. 2022, 144, 2085–2089.
- 14 Bai, R.; Zhang, Z.; Di, W.; Yang, X.; Zhao, J.; Ouyang, H.; Liu, G.; Zhang, X.; Cheng, L.; Cao, Y.; Yu, W.; Yan, X. Oligo[2]catenane That Is Robust at Both the Microscopic and Macroscopic Scales. J. Am. Chem. Soc. 2023, 145, 9011–9020.
- 15 Olson, M. A.; Coskun, A.; Fang, L.; Basuray, A. N.; Stoddart, J. F. Polycatenation under Thermodynamic Control. Angew. Chem. Int. Ed. 2010, 49, 3151–3156.
- 16 Wu, S.; Cai, C.; Li, F.; Tan, Z.; Dong, S. Deep Eutectic Supramolecular Polymers: Bulk Supramolecular Materials. Angew. Chem. Int. Ed. 2020, 59, 11871–11875.
- 17 Xing, H.; Li, Z.; Wu, Z. L.; Huang, F. Catenane Crosslinked Mechanically Adaptive Polymer Gel. Macromol. Rapid Commun. 2018, 39, 1700361.
- 18 Hart, L. F.; Lenart, W. R.; Hertzog, J. E.; Oh, J.; Turner, W. R.; Dennis, J. M.; Rowan, S. J. Doubly Threaded Slide-Ring Polycatenane Networks. J. Am. Chem. Soc. 2023, 145, 12315–12323.
- 19 Liu, X.; Tang, J.; Chen, Q.; Wang, L. Structurally Switchable Hydrogels with Multifunctions Enabled by Controlled Catenated Junctions. Macromolecules 2024, 57, 174–180.
- 20 Li, J.; Hu, Z.; Xu, S.; Quan, X.; Ji, X. Self-assembled poly[2]catenanes based on non-covalent and dynamic covalent bonds. Polym. Chem. 2024, 15, 1786–1793.
- 21 Aricó, F.; Chang, T.; Cantrill, S. J.; Khan, S. I.; Stoddart, J. F. Template-Directed Synthesis of Multiply Mechanically Interlocked Molecules Under Thermodynamic Control. Chem. Eur. J. 2005, 11, 4655–4666.
- 22 Chen, Q.; Li, Z.; Lei, Y.; Chen, Y.; Tang, H.; Wu, G.; Sun, B.; Wei, Y.; Jiao, T.; Zhang, S.; Huang, F.; Wang, L.; Li, H. The sharp structural switch of covalent cages mediated by subtle variation of directing groups. Nat. Commun. 2023, 14, 4627.
- 23 Chen, Y.; Cao, Z.; Feng, T.; Zhang, X.; Li, Z.; Dong, X.; Huang, S.; Liu, Y.; Cao, X.; Sue, A. C. H.; Peng, C.; Lin, X.; Wang, L.; Li, H. Enantioselective Self-Assembly of a Homochiral Tetrahedral Cage Comprising Only Achiral Precursors. Angew. Chem. Int. Ed. 2024, 63, e202400467.
- 24 Chen, Q.; Lei, Y.; Wu, G.; Li, Q.; Pan, Y.; Li, H. Ultramacrocyclization in water via external templation. Chem. Sci. 2022, 13, 798–803.
- 25
Wu, M.; Han, L.; Yan, B.; Zeng, H. Self-healing hydrogels based on reversible noncovalent and dynamic covalent interactions: A short review. Supramol. Mater. 2023, 2, 100045.
10.1016/j.supmat.2023.100045 Google Scholar
- 26 Su, G.; Li, Z.; Gong, J.; Zhang, R.; Dai, R.; Deng, Y.; Tang, B. Z. Information-Storage Expansion Enabled by a Resilient Aggregation-Induced-Emission-Active Nanocomposite Hydrogel. Adv. Mater. 2022, 34, 2207212.
- 27 Yan, X.; Xu, D.; Chi, X.; Chen, J.; Dong, S.; Ding, X.; Yu, Y.; Huang, F. A Multiresponsive, Shape-Persistent, and Elastic Supramolecular Polymer Network Gel Constructed by Orthogonal Self-Assembly. Adv. Mater. 2012, 24, 362–369.
- 28 Leigh, D. A.; Morales, M. Á. F.; Pérez, E. M.; Wong, J. K. Y.; Saiz, C. G.; Slawin, A. M. Z.; Carmichael, A. J.; Haddleton, D. M.; Brouwer, A. M.; Buma, W. J.; Wurpel, G. W. H.; León, S.; Zerbetto, F. Patterning through Controlled Submolecular Motion: Rotaxane-Based Switches and Logic Gates that Function in Solution and Polymer Films. Angew. Chem. Int. Ed. 2005, 44, 3062–3067.
- 29 Ahmadi, M.; Seiffert, S. Dynamic Model Metallo-Supramolecular Dual-Network Hydrogels with Independently Tunable Network Crosslinks. J. Polym. Sci. 2020, 58, 330–342.
- 30
Zhang, H.; Liu, H.; Hu, Z.; Ji, X. Multicolor fluorescent supramolecular adhesive gels based on a single molecule with aggregation-induced ratiometric emission. Supramol. Mater. 2022, 1, 100018.
10.1016/j.supmat.2022.100018 Google Scholar
- 31 Zhao, J.; Zhang, Z.; Wang, C.; Yan, X. Synergistic Dual Dynamic Bonds in Covalent Adaptable Networks. CCS Chem. 2024, 6, 41–56.
- 32 Zhang, M.; Xu, D.; Yan, X.; Chen, J.; Dong, S.; Zheng, B.; Huang, F. Self-Healing Supramolecular Gels Formed by Crown Ether Based Host–Guest Interactions. Angew. Chem. Int. Ed. 2012, 51, 7011–7015.




