One-Dimensional Metal-Organic Framework for High-Efficiency Electrocatalytic Reduction of CO2 to CO†
Jie Lu
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorQianyu Wang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorZhikai Jin
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYang Xiao
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorBi-Hong Huang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCai-Hong Zhang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorGui-Zeng Yang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYi Zhou
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Fu-Sheng Ke
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]Search for more papers by this authorJie Lu
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorQianyu Wang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorZhikai Jin
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYang Xiao
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorBi-Hong Huang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCai-Hong Zhang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorGui-Zeng Yang
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorYi Zhou
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Fu-Sheng Ke
Hubei Key Laboratory of Electrochemical Power Sources, Hubei Key Lab on Organic and Polymeric Opto-Electronic Materials, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
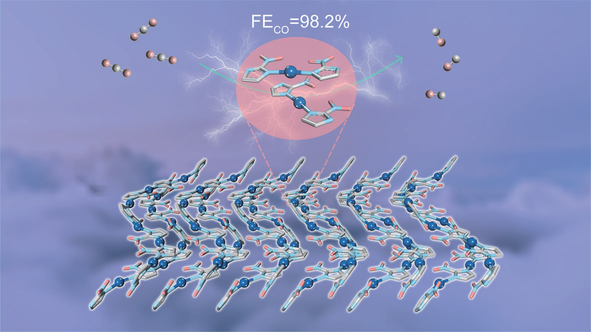
Electrocatalytic reduction of CO2 to valuable products possesses huge potential to alleviate environmental and energy crisis. It is well known that Ag favors the conversion of CO2 to CO but the exposed active sites and stability are still rather limited. In this study, a novel one-dimensional Ag-based metal-organic framework (1D Ag-NIM-MOF) was successfully synthesized and used in the electrocatalytic CO2 reduction reaction (CO2RR) for the first time. As a result, the Faradaic efficiency of CO achieved 94.5% with current density of 12.5 mA·cm–2 in an H-type cell and 98.2% with current density of 161 mA·cm–2 in a flow cell at –1.0 V (vs. RHE), which stands as a new benchmark of Ag-based MOFs in the electrocatalytic CO2RR. The excellent performance of 1D Ag-NIM-MOF is attributed to its peculiar one-dimensional structure, which is beneficial for diffusion of reactants and products, and exposure of much more catalytic sites. Compared to commercial Ag nanoparticles, 1D Ag-NIM-MOF exhibits superior electrocatalytic CO2RR performance with higher catalytic activity and stability.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400420-sup-0001-supinfo.pdfPDF document, 2.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Lai, W.; Qiao, Y.; Zhang, J.; Lin, Z.; Huang, H. Design strategies for markedly enhancing energy efficiency in the electrocatalytic CO2 reduction reaction. Energy Environ. Sci. 2022, 15, 3603–3629.
- 2 Zhang, Y.; Wang, C.; Shu, W. Research progress of carbon dioxide reduction and utilization. Chem. Ind. Eng. Prog. 2023, 42, 944–956.
- 3 Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129.
- 4 Zheng, T.; Jiang, K.; Wang, H. Recent Advances in Electrochemical CO2 to CO Conversion on Heterogeneous Catalysts. Adv. Mater. 2018, 30, 1802066.
- 5 Jones, J.; Prakash, G.; Olah, G. Electrochemical CO2 Reduction: Recent Advances and Current Trends. Isr. J. Chem. 2014, 54, 1451–1466.
- 6 Zhu, D.; Liu, J.; Qiao, S. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 2016, 28, 3423–3452.
- 7
Lugo-Morin, D. Global future: Low-carbon economy or high-carbon economy? World 2021, 2, 175–193.
10.3390/world2020012 Google Scholar
- 8 Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675.
- 9 Perfecto-Irigaray, M.; Merino-Garcia, I.; Albo, J.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S. Copper(II)-porphyrin functionalized titanium(IV) metal-oganic aerogels for the visible-light driven conversion of CO2 to alcohols. Mater. Today Energy 2023, 36, 101346.
- 10 Angulo-Ibáñez, A.; Perfecto-Irigaray, M.; Merino-Garcia, I.; Luengo, N.; Goitandia, A.; Albo, J.; Aranzabe, E.; Beobide, G.; Castillo, O.; Pérez-Yáñez, S. Metal-organic aerogels based on titanium(IV) for visible-light conducted CO2 photoreduction to alcohols. Mater. Today Energy 2022, 30, 101178.
- 11 Sanz-Pérez, E.; Murdock, C.; Didas, S.; Jone, C. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876.
- 12 Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648.
- 13 Han, G. H.; Bang, J.; Park, G.; Choe, S.; Jang, Y. J.; Jang, H. W.; Kim, S. Y.; Ahn, S. H. Recent advances in electrochemical, photochemical, and photoelectrochemical reduction of CO2 to C2+ products. Small 2023, 19, 2205765.
- 14 Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 conversion: From fundamentals to value-added products. Chem. Soc. Rev. 2021, 50, 4993–5061.
- 15 Zhao, J.; Xue, S.; Barber, J.; Zhou, Y.; Meng, J.; Ke, X. An overview of Cu-based heterogeneous electrocatalysts for CO2 reduction. J. Mater. Chem. A 2020, 8, 4700–4734.
- 16 Popović, S.; Smiljanić, M.; Jovanovič, P.; Vavra, J.; Buonsanti, R.; Hodnik, N. Stability and degradation mechanisms of copper-based catalysts for electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2020, 59, 14736–14746.
- 17 Sheng, W.; Kattel, S.; Yao, S.; Yan, B.; Liang, Z.; Hawxhurst, C.; Wu, Q.; Chen, J. Electrochemical reduction of CO2 to synthesis gas with controlled CO/H2 ratios. Energy Environ. Sci. 2017, 10, 1180–1185.
- 18 Yang, H.; Huang, J.; Yang, H.; Guo, Q.; Jiang, B.; Chen, J.; Yuan, X. Design and synthesis of Ag-based catalysts for electrochemical CO2 reduction: Advances and perspectives. Chem. Asian J. 2022, 17, e202200637.
- 19 Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc., Faraday Trans. 1989, 85, 2309–2326.
- 20
Chen, Y.; Li, C.; Kanan, M. Aqueous CO2 Reduction at Very Low Overpotential on Oxide-Derived Au Nanoparticles. J. Am. Chem. Soc. 2012, 49, 19969–19972.
10.1021/ja309317u Google Scholar
- 21
Mistry, H.; Reske, R.; Zeng, Z.; Zhao, Z.; Greeley, J.; Strasser, P.; Cuenya, B. Exceptional Size-Dependent Activity Enhancement in the Electroreduction of CO2 over Au Nanoparticles. J. Am. Chem. Soc. 2014, 47, 16473–16476.
10.1021/ja508879j Google Scholar
- 22 Zhao, S.; Jin, R.; Jin, R. Opportunities and challenges in CO2 reduction by gold- and silver-based electrocatalysts: From bulk metals to nanoparticles and atomically precise nanoclusters. ACS Energy Lett. 2018, 3, 452–462.
- 23 Chakrabarti, S.; Woods, T. J.; Mirica, L. M. Insights into the Mechanism of CO2 Electroreduction by Molecular Palladium-Pyridinophane Complexes. Inorg. Chem. 2023, 62, 16801–16809.
- 24 Zeng, L.; Shi, J.; Chen, H.; Lin, C. Ag Nanowires/C as a Selective and Efficient Catalyst for CO2 Electroreduction. Energies 2021, 14, 2840.
- 25 Zou, Y.; Zhan, T.; Yang, Y.; Fan, Z.; Li, Y.; Zhang, Y.; Ma, X.; Chen, Q.; Xiang, S.; Zhang, Z. Single-phase proton- and electron-conducting Ag-organic coordination polymers for efficient CO2 Electroreduction. J. Mater. Chem. A 2022, 10, 3216–3225.
- 26 Zhao, S.; Zhu, D.; Peng, X.; Song, W. An empirical analysis of the regional competitiveness based on S&T talents flow. Nat. Energy 2016, 1, 1–10.
- 27 Guo, G.; Jiao, Y.; Zheng, Y.; Luo, J.; Davey, K.; Qiao, S. Intermediate Modulation on Noble Metal Hybridized to 2D Metal-Organic Framework for Accelerated Water Electrocatalysis. Chem 2019, 5, 2429–2441.
- 28 Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663.
- 29 Diercks, C. S.; Liu, Y.; Cordova, K. E.; Yaghi, O. M. The Role of Reticular Chemistry in the Design of CO2 Reduction Catalysts. Nat. Mater. 2018, 17, 301–307.
- 30 Nam, D.-H.; De Luna, P.; Rosas-Hernández, A.; Thevenon, A.; Li, F.; Agapie, T.; Peters, J. C.; Shekhah, O.; Eddaoudi, M.; Sargent, E. H. Molecular Enhancement of Heterogeneous CO2 Reduction. Nat. Mater. 2020, 19, 266–276.
- 31 Hou, S.-L.; Dong, J.; Zhao, B. Formation of C-X Bonds in CO2 Chemical Fixation Catalyzed by Metal-Organic Frameworks. Adv. Mater. 2020, 32, 1806163.
- 32 Albo, J.; Vallejo, D.; Beobide, G.; Castillo, O.; Castaño, P.; Irabien, A. Copper-Based Metal-Organic Porous Materials for CO2 Electrocatalytic Reduction to Alcohols. ChemSusChem. 2017, 10, 1100–1109.
- 33 Albo, J.; Perfecto-Irigaray, M.; Beobide, G.; Irabien, A. Cu/Bi Metal-Organic Framework-Based Systems for an Enhanced Electrochemical Transformation of CO2 to Alcohols. J. CO2 Util. 2019, 33, 157–165.
- 34
Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal-Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373.
10.1002/advs.201802373 Google Scholar
- 35 Liu, J.; Xie, Y.; Gao, Q.; Cao, F.; Qin, L.; Wu, Z.; Zhang, W.; Li, H.; Zhang, C. 1D MOF-Derived N-Doped Porous Carbon Nanofibers Encapsulated with Fe3C Nanoparticles for Efficient Bifunctional Electrocatalysis. Eur. J. Inorg. Chem. 2020, 2020, 581–589.
- 36 Khiarak, B.; Hasanzadeh, M.; Mojaddami, M.; Far, H.; Simchi, A. In situ synthesis of quasi-needle-like bimetallic organic frameworks on highly porous graphene scaffolds for efficient electrocatalytic water oxidation. Chem. Commun. 2020, 56, 3135–3138.
- 37 Landaluce, N.; Perfecto-Irigaray, M.; Albo, J.; Beobide, G.; Castillo, O.; Irabien, A.; Luque, A.; Méndez, A. S. J.; Platero-Prats, A. E.; Pérez-Yáñez, S. Copper(II) invigorated EHU-30 for continuous electroreduction of CO2 into value–added chemicals. Sci. Rep. 2022, 12, 8505.
- 38 Perfecto-Irigaray, M.; Albo, J.; Beobide, G.; Castillo, O.; Irabienb, A.; Pérez-Yáñez, S. Synthesis of heterometallic metal–organic frameworks and their performance as electrocatalyst for CO2 reduction. RSC Adv. 2018, 8, 21092–21099.
- 39 Cai, X.; Xie, Z.; Li, D.; Kassymova, M.; Zang, S.; Jiang, H. Nano-size metal-organic frameworks: Synthesis and applications. Coord. Chem. Rev. 2020, 417, 213366.
- 40 Zhang, J.; Zhang, Y.; Lin, J.; Chen, X. Metal Azolate Frameworks: From Crystal Engineering to Functional Materials. Chem. Rev. 2012, 112, 1001–1033.
- 41 Huang, D.; Zhu, H.; Zhao, Z.; Huang, J.; Liao, P.; Chen, X. A Stable and Low-Cost Metal-Azolate Framework with Cyclic Tricopper Active Sites for Highly Selective CO2 Electroreduction to C2+ Products. ACS Catal. 2022, 12, 8444–8450.
- 42
Zhuo, L.; Chen, P.; Zheng, K.; Zhang, X.; Wu, J.; Lin, D.; Liu, S.; Wang, Z.; Liu, J.; Zhou, D.; Zhang, J. Flexible Cuprous Triazolate Frameworks as Highly Stable and Efficient Electrocatalysts for CO2 Reduction with Tunable C2H4/CH4 Selectivity. Angew. Chem. Int. Ed. 2022, 134, e202204967.
10.1002/ange.202204967 Google Scholar
- 43 Huang, X.; Zhang, J.; Chen, X. A New Route to Supramolecular Isomers via Molecular Templating: Nanosized Molecular Polygons of Copper(I) 2-Methylimidazolates. J. Am. Chem. Soc. 2004, 126, 13218–13219.
- 44 Huang, X.; Li, D.; Chen, X. Solvent-induced supramolecular isomerism in silver(I) 2-methylimidazolate. CrystEngComm 2006, 8, 351–355.
- 45 Zou, Y.; Pan, T.; Fan, Z.; Li, Y.; Zhang, H.; Ju, Y.; Zhang, Y.; Ma, X.; Chen, Q.; Xiang, S.; Zhang, Z. Ag-organic coordination polymers with multi-dimensional electron transfer channels for enhancing CO2 electroreduction. Chem. Eng. J. 2023, 454, 140496.
- 46 Yoshida, T. An X-Ray Photoelectron Spectroscopic Study of Several Metal Complexes of 2-Mercaptobenzimidazole and 2-Mercaptobenzoxazole. Bull. Chem. Soc. Jpn. 1980, 53, 1449–1450.
- 47 Sharma, J.; Garrett, W.; Owens, F.; Vogel, V. X-ray photoelectron study of electronic structure, ultraviolet, and isothermal decomposition of 1,3,5-triamino-2,4,6-trinitrobenzene. J. Phys. Chem. 1982, 86, 1657–1661.
- 48
Yan, W.; Mo, Q.; He, Q.; Li, X.; Xue, Z.; Lu, Y.; Chen, J.; Zheng, K.; Fan, Y.; Li, G.; Su, C. Anion Modulation of Ag-Imidazole Cuboctahedral Cage Microenvironments for Efficient Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2024, https://doi.org/10.1002/anie.202406564.
10.1002/anie.202406564 Google Scholar




