Enantioselective Construction of Polycyclic Chromanes through Organocatalytic Sequential Quintuple Reaction via One-Pot Step-Wise Procedure
Jie Wang
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Search for more papers by this authorHang Qin
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Search for more papers by this authorYa-Li Song
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Baoding, Hebei, 071002 China
Search for more papers by this authorFei Cao
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Baoding, Hebei, 071002 China
Search for more papers by this authorCorresponding Author
Zhi-Hao You
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Baoding, Hebei, 071002 China
E-mail: [email protected]Search for more papers by this authorJie Wang
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Search for more papers by this authorHang Qin
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Search for more papers by this authorYa-Li Song
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Baoding, Hebei, 071002 China
Search for more papers by this authorFei Cao
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Baoding, Hebei, 071002 China
Search for more papers by this authorCorresponding Author
Zhi-Hao You
Key Laboratory of Pharmaceutical Quality Control of Hebei Province, College of Pharmaceutical Sciences, Hebei University, Baoding, Hebei, 071002 China
Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Baoding, Hebei, 071002 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
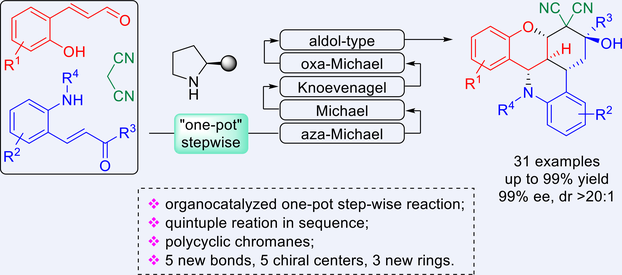
An efficient and highly stereoselective synthetic method to access polycyclic chromanes has been achieved through organocatalyzed one-pot step-wise reactions involving 2-hydroxycinnamaldehydes, 2-aminochalcones, and malononitrile as substrates. The reactions underwent a quintuple process by aza-Michael/Michael/Knoevenagel/oxa-Michael/aldol-type reaction in sequence to give products bearing 3 new generated rings and 5 chiral centers in moderate to quantitative yields with excellent stereoselectivities. A novel retro-reaction mechanism was discovered in the synthetic transformations of products.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400328-sup-0001-supinfo.pdfPDF document, 11.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Poulin, J.; Grisé-Bard, C. M.; Barriault, L. Pericyclic Domino Reactions: Concise Approaches to Natural Carbocyclic Frameworks. Chem. Soc. Rev. 2009, 38, 3092–3101; (b) Volla, C. M. R.; Atodiresei, I.; Rueping, M. Catalytic C–C Bond-Forming Multi-Component Cascade or Domino Reactions: Pushing the Boundaries of Complexity in Asymmetric Organocatalysis. Chem. Rev. 2014, 114, 2390–2431; (c) Hayashi, Y. Pot Economy and One-pot Synthesis. Chem. Sci. 2016, 7, 866–880; (d) Klier, L.; Tur, F.; Poulsen, P. H.; Jørgensen, K. A. Asymmetric Cycloaddition Reactions Catalysed by Diarylprolinol Silyl Ethers. Chem. Soc. Rev. 2017, 46, 1080–1102.
- 2For selected reviews, see: (a) Nicolaou, K. C.; Montagnon, T.; Snyder, S. A. Tandem Reactions, Cascade Sequences, and Biomimetic Strategies in Total Synthesis. Chem. Commun. 2003, 5, 551–564; (b) Nicolaou, K. C.; Chen, J. S. The Art of Total Synthesis Through Cascade Reactions. Chem. Soc. Rev. 2009, 38, 2993–3009; (c) Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic Cascade Reactions as a New Tool in Total Synthesis. Nat. Chem. 2010, 2, 167–178; (d) Smith, J. M.; Moreno, J.; Boal, B. W.; Garg, N. K. Cascade Reactions: A Driving Force in Akuammiline Alkaloid Total Synthesis. Angew. Chem. Int. Ed. 2015, 54, 400−412; (e) Ardkhean, R.; Caputo, D. F. J.; Morrow, S. M.; Shi, H.; Xiong, Y.; Anderson, E. A. Cascade Polycyclizations in Natural Product Synthesis. Chem. Soc. Rev. 2016, 45, 1557–1569; (f) Jiang, Y.; McNamee, R. E.; Smith, P. J.; Sozanschi, A.; Tong, Z.; Anderson, E. A. Advances in Polycyclization Cascades in Natural Product Synthesis. Chem. Soc. Rev. 2021, 50, 58–71; (g) Holman, K. R.; Stanko, A. M.; Reisman, S. E. Palladium-catalyzed Cascade Cyclizations Involving C–C and C–X Bond Formation: Strategic Applications in Natural Product Synthesis. Chem. Soc. Rev. 2021, 50, 7891–7908. For selected examples, see: (h) Davidson, S. J.; Barker, D. Total Synthesis of Ovafolinins A and B: Unique Polycyclic Benzoxepin Lignans through a Cascade Cyclization. Angew. Chem. Int. Ed. 2017, 56, 9483–9486; (i) Liu, W.; Qin, W.; Wang, X.; Xue, F.; Liu, X.-Y.; Qin, Y. Bioinspired Synthesis of (+)-Cinchonidine Using Cascade Reactions. Angew. Chem. Int. Ed. 2018, 57, 12299–12302; (j) He, L.; Wang, X.; Wu, X.; Meng, Z.; Peng, X.; Liu, X.-Y.; Qin, Y. Asymmetric Total Synthesis of (+)-Strychnine. Org. Lett. 2019, 21, 252–255; (k) Li, L.-X.; Min, L.; Yao, T.-B.; Ji, S.-X.; Qiao, C.; Tian, P.-L.; Sun, J.; Li, C.-C. Total Synthesis of Yuzurine-type Alkaloid Daphgraciline. J. Am. Chem. Soc. 2022, 144, 18823–18828.
- 3(a) Roll, D. M.; Manning, J. K.; Carter, G. T. Hongoquercins A and B, New Sesquiterpenoid Antibiotics: Isolation, Structure Elucidation, and Antibacterial Activity. J. Antibiot. 1998, 51, 635–639; (b) Balbin-Oliveros, M.; Edrada, R. A.; Proksch, P.; Wray, V.; Witte, L.; Van Soest, R. W. M. A New Meroditerpenoid Dimer from an Undescribed Philippine Marine Sponge of the Genus Strongylophora. J. Nat. Prod. 1998, 61, 948–952; (c) Nakatani, M.; Nakamura, M.; Suzuki, A.; Inoue, M.; Katoh, T. A New Strategy toward the Total Synthesis of Stachyflin, A Potent Anti-Influenza A Virus Agent: Concise Route to the Tetracyclic Core Structure. Org. Lett. 2002, 4, 4483–4486; (d) Trost, B. M.; Shen, H. C.; Surivet, J.-P. Biomimetic Enantioselective Total Synthesis of (−)-Siccanin via the Pd-Catalyzed Asymmetric Allylic Alkylation (AAA) and Sequential Radical Cyclizations. J. Am. Chem. Soc. 2004, 126, 12565–12579; (e) Mohammed, K. A.; Jadulco, R. C.; Bugni, T. S.; Harper, M. K.; Sturdy, M.; Ireland, C. M. Strongylophorines: Natural Product Inhibitors of Hypoxia-Inducible Factor-1 Transcriptional Pathway. J. Med. Chem. 2008, 51, 1402–1405; (f) Liron, F.; Fontana, F.; Zirimwabagabo, J.-O.; Prestat, G.; Rajabi, J.; Rosa, C. L.; Poli, G. A New Cross-Coupling-Based Synthesis of Carpanone. Org. Lett. 2009, 11, 4378–4381; (g) Barrett, T. N.; Barrett, A. G. M. Cascade Polyketide and Polyene Cyclizations: Biomimetic Total Synthesis of Hongoquercin B. J. Am. Chem. Soc. 2014, 136, 17013–17015.
- 4For selected reviews, see: (a) Pratap, R.; Ram, V. J. Natural and Synthetic Chromenes, Fused Chromenes, and Versatility of Dihydrobenzo[h]chromenes in Organic Synthesis. Chem. Rev. 2014, 114, 10476–10526; (b) Desimoni, G.; Faita, G.; Quadrelli, P. Forty Years after “Heterodiene Syntheses with α,β-Unsaturated Carbonyl Compounds”: Enantioselective Syntheses of 3,4-Dihydropyran Derivatives. Chem. Rev. 2018, 118, 2080–2248. For selected examples, see: (c) Kumar, M.; Chauhan, P.; Valkonen, A.; Rissanen, K.; Enders, D. Asymmetric Synthesis of Functionalized Tricyclic Chromanes via an Organocatalytic Triple Domino Reaction. Org. Lett. 2017, 19, 3025–3028; (d) You, Z.-H.; Chen, Y.-H.; Tang, Y.; Liu, Y.-K. Organocatalytic Asymmetric Synthesis of Spiro-Bridged and Spiro-Fused Heterocyclic Compounds Containing Chromane, Indole, and Oxindole Moieties. Org. Lett. 2018, 20, 6682–6686; (e) Kumar, M.; Chauhan, P.; Bailey, S. J.; Jafari, E.; von Essen, C.; Rissanen, K.; Enders, D. Organocatalytic Oxa-Michael/Michael/Michael/Aldol Condensation Quadruple Domino Sequence: Asymmetric Synthesis of Tricyclic Chromanes. Org. Lett. 2018, 20, 1232–1235; (f) Xiang, M.; Li, C.-Y.; Song, X.-J.; Zou, Y.; Huang, Z.-C.; Li, X.; Tian, F.; Wang, L.-X. Organocatalytic and Enantioselective [4+2] Cyclization between Hydroxymaleimides and ortho-Hydroxyphenyl para-Quinone Methide-Selective Preparation of Chiral Hemiketals. Chem. Commun. 2020, 56, 14825–14828; (g) Zhu, Z.; Odagi, M.; Supantanapong, N.; Xu, W.; Saame, J.; Kirm, H.-U.; Abboud, K. A.; Leito, I.; Seidel, D. Modular Design of Chiral Conjugate-Base-Stabilized Carboxylic Acids: Catalytic Enantioselective [4 + 2] Cycloadditions of Acetals. J. Am. Chem. Soc. 2020, 142, 15252–15258; (h) Zhu, X.-Q.; Wang, Q.; Zhu, J. Organocatalytic Enantioselective Diels–Alder Reaction of 2-Trifluoroacetamido-1,3-dienes with α,β-Unsaturated Ketones. Angew. Chem. Int. Ed. 2023, 62, DOI: https://doi.org/10.1002/anie.202214925.
- 5 You, Z.-H.; Chen, Y.-H.; Tang, Y.; Liu, Y.-K. Application of E1cB Elimination in Asymmetric Organocatalytic Cascade Reactions To Construct Polyheterocyclic Compounds. Org. Lett. 2019, 21, 8358–8363.
- 6 You, Z.-H.; Liu, Y.-K. Asymmetric Organocatalytic Access to Spiro-fused Heterocyclic Compounds: E1cB Elimination Mediates Formal [4 + 2] Annulation. Org. Lett. 2022, 24, 6288–6291.
- 7(a) Lv, X.-J.; Chen, Y.-H.; Liu, Y.-K. Two Competitive but Switchable Organocatalytic Cascade Reaction Pathways: The Diversified Synthesis of Chiral Acetal-Containing Bridged Cyclic Compounds. Org. Lett. 2019, 21, 190–195; (b) Chen, Y.-H.; Lv, X.-J.; You, Z.-H.; Liu, Y.-K. Asymmetric Organocatalyzed Reaction Sequence of 2-Hydroxy Cinnamaldehydes and Acyclic N-Sulfonyl Ketimines to Construct Diverse Chiral Bridged Polycyclic Aminals. Org. Chem. Front. 2019, 6, 3725–3730; (c) Chen, Y.-H.; Lv, X.-J.; You, Z.-H.; Liu, Y.-K. Asymmetric Organocatalyzed Reaction Sequence to Synthesize Chiral Bridged and Spiro-Bridged Benzofused Aminals via Divergent Pathways. Org. Lett. 2019, 21, 5556–5561; (d) Wang, C.; Chen, Y.-H.; Wu, H.-C.; Wang, C.; Liu, Y.-K. The Quinary Catalyst–Substrate Complex Induced Construction of Spiro-Bridged or Cagelike Polyheterocyclic Compounds via a Substrate-Controlled Cascade Process. Org. Lett. 2019, 21, 6750–6755; (e) Zhang, X.-Q.; Lv, X.-J.; Pei, J.-P.; Tan, R.; Liu, Y.-K. An Asymmetric Multicatalytic Reaction Sequence of 2-Hydroxycinnamaldehydes and Enolic 1,3-Dicarbonyl Compounds to Construct Bridged Bicyclic Acetals. Org. Chem. Front. 2020, 7, 292–297; (f) Vachan, B. S.; Karuppasamy, M.; Jan, G.; Bhuvanesh, N.; Maheswari, C. U.; Sridharan, V. Direct Access to Bridged Tetrahydroquinolines and Chromanes via an InCl3-Catalyzed Sequential Three-Component Cascade. J. Org. Chem. 2020, 85, 8062–8073; (g) Peng, C.-J.; Pei, J.-P.; Chen, Y.-H.; Wu, Z.-Y.; Liu, M.; Liu, Y.-K. Enantioselective Organocatalytic Sequential Michael-Cyclization of Functionalized Nitroalkanes to 2-Hydroxycinnamaldehydes: Synthesis of Benzofused Dioxa[3.3.1] and Oxa[4.3.1] Methylene-Bridged Compounds. Org. Chem. Front. 2021, 8, 4217–4223; (h) Ming, Y.-C.; Lv, X.-J.; Liu, M.; Liu, Y.-K. Synthesis of Chiral Polycyclic Tetrahydrocarbazoles by Enantioselective Aminocatalytic Double Activation of 2-Hydroxycinnamaldehydes with Dienals. Org. Lett. 2021, 23, 6515–6519; (i) Ming, Y.-C.; Lv, X.-J.; Chen, Y.-H.; Liu, Y.-K. Asymmetric Iminium Ion-Catalyzed Conjugate Addition of 2-Hydroxycinnamaldehydes and 2-Oxocarboxylic Esters: Synthesis of Chiral Polysubstituted Bridged Bicyclic Ketals. Chem. Commun. 2023, 59, 8711–8714.
- 8For selected reviews, see: (a) Muthukrishnan, I.; Sridharan, V.; Menéndez, J. C. Progress in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2019, 119, 5057–5191; (b) Song, Y.-X.; Du, D.-M. Recent Advances in Catalytic Asymmetric Aza-Michael Addition Triggered Cascade Reactions. Adv. Synth. Catal. 2021, 363, 4667–4694. For selected examples, see: (c) Zhang, H.-R.; Dong, Z.-W.; Yang, Y.-J.; Wang, P.-L.; Hui, X.-P. N-Heterocyclic Carbene-Catalyzed Stereoselective Cascade Reaction: Synthesis of Functionalized Tetrahydroquinolines. Org. Lett. 2013, 15, 4750–4753; (d) Zhang, Q.-L.; Jin, H.-X.; Feng, J.-X.; Zhu, Y.-N.; Jia, P.-H.; Wu, C.-Z.; Huang, Y. Sequential Phosphine-Catalyzed [4 + 2] Annulation of β’-Acetoxy Allenoates: Enantioselective Synthesis of 3-Ethynyl-Substituted Tetrahydroquinolines. Org. Lett. 2019, 21, 1407–1411; (e) Zhang, J.-L.; Ma, R.; Zhao, H.-H.; Xu, P.-F. Enantioselective Construction of Spiro-Tetrahydroquinoline Scaffolds through Asymmetric Catalytic Cascade Reactions. Chem. Commun. 2022, 58, 3493–3496.
- 9(a) Tian, J.-M.; Yuan, Y.-H.; Xie, Y.-Y.; Zhang, S.-Y.; Ma, W.-Q.; Zhang, F.-M.; Wang, S.-H.; Zhang, X.-M.; Tu, Y.-Q. Catalytic Asymmetric Cascade Using Spiro-Pyrrolidine Organocatalyst: Efficient Construction of Hydrophenanthridine Derivatives. Org. Lett. 2017, 19, 6618–6621; (b) Mukherjee, S.; Shee, S.; Poisson, T.; Besset, T.; Biju, A. T. Enantioselective N-Heterocyclic Carbene-Catalyzed Cascade Reaction for the Synthesis of Pyrroloquinolines via N–H Functionalization of Indoles. Org. Lett. 2018, 20, 6998–7002; (c) Zeng, R.; Li, J.-L.; Zhang, X.; Liu, Y.-Q.; Jia, Z.-Q.; Leng, H.-J.; Huang, Q.-W.; Liu, Y.; Li, Q.-Z. Diastereoselective Construction of 6,8-Dioxabicyclo[3.2.1]octane Frameworks from Vinylethylene Carbonates via Palladium-Organo Relay Catalysis. ACS Catal. 2019, 9, 8256–8262; (d) Zhou, X.-J.; Zhao, J.-Q.; Chen, X.-M.; Zhuo, J.-R.; Zhang, Y.-P.; Chen, Y.-Z.; Zhang, X.-M.; Xu, X.-Y.; Yuan, W.-C. Organocatalyzed Asymmetric Dearomative Aza-Michael/Michael Addition Cascade of 2-Nitrobenzofurans and 2-Nitrobenzothiophenes with 2-Aminochalcones. J. Org. Chem. 2019, 84, 4381–4391; (e) Ma, R.-Y.; Yang, H.-Z.; Ye, L.; Yang, Q.; Shi, Z.-C.; Zhao, Z.-G.; Li, X.-F. Stereoselective Synthesis of Chiral Hydrophenanthridines via a One-Pot Stepwise Aza-Michael/Michael/Michael Process. Org. Lett. 2022, 24, 4798–4803; (f) Zhu, J.-X.; Pi, F.; Sun, T.; Huang, W.-Y.; Gao, L.; Chen, Z.-C.; Du, W.; Chen, Y.-C. Asymmetric 2,4-Dienylation/[4 + 2] Annulation Cascade to Construct Fused Frameworks via Auto-Tandem Palladium Catalysis. Org. Lett. 2023, 25, 3682–3686.
- 10(a) Zhang, J.-L.; Liu, J.-Y.; Xu, G.-Q.; Luo, Y.-C.; Lu, H.; Tan, C.-Y.; Hu, X.-Q.; Xu, P.-F. One-Pot Enantioselective Construction of Polycyclic Tetrahydroquinoline Scaffolds through Asymmetric Organo/Photoredox Catalysis via Triple-Reaction Sequence. Org. Lett. 2021, 23, 3287–3293; (b) Zhang, J.-L.; Ye, W.-L.; Zhang, J.; Hu, X.-Q.; Xu, P.-F. Enantioselective Construction of Polycyclic Indazole Skeletons Bearing Five Consecutive Chiral Centers through an Asymmetric Triple-Reaction Sequence. Org. Lett. 2021, 23, 5033–5038; (c) Zhang, J.-L.; He, W.-B.; Hu, X.-Q.; Xu, P.-F. New Strategies for Asymmetric Photocatalysis: Asymmetric Organocatalytic/Photoredox Relay Catalysis for Efficient Synthesis of Polycyclic Compounds Containing Vicinal Amino Alcohols. Sci. China Chem. 2024, 67, 945–952.




