Visible-Light-Induced Domino Cyclization to Access Pyrido[2,3-d]pyrimidine-2,4-diones via a Radical-Polar Crossover Reaction
Wanqing Zuo
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorYu Cheng
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
Search for more papers by this authorZhizhen Zhu
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
Search for more papers by this authorLingling Zuo
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
Search for more papers by this authorCorresponding Author
Xiao Geng
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhifang Li
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorCorresponding Author
Lei Wang
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
E-mail: [email protected]; [email protected]Search for more papers by this authorWanqing Zuo
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorYu Cheng
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
Search for more papers by this authorZhizhen Zhu
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
Search for more papers by this authorLingling Zuo
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
Search for more papers by this authorCorresponding Author
Xiao Geng
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhifang Li
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
Search for more papers by this authorCorresponding Author
Lei Wang
Advanced Research Institute and School of Pharmaceutical Sciences, Taizhou University, Jiaojiang, Zhejiang, 318000 China
College of Material Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
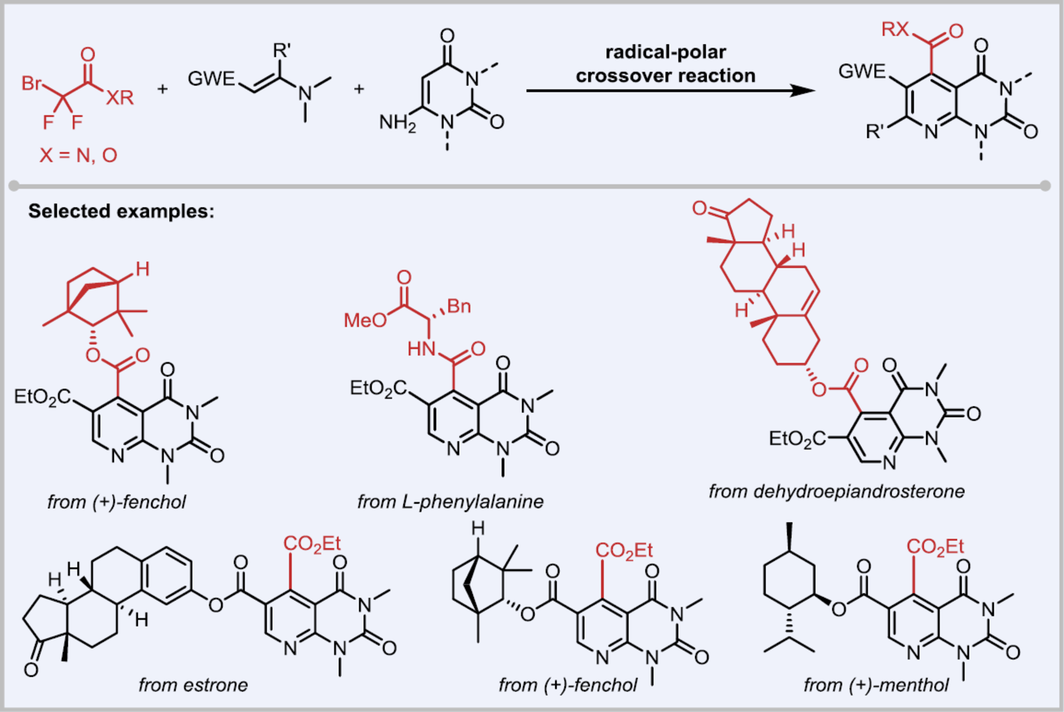
Catalytic and green strategies for the synthesis of privileged scaffolds are synthetically appealing. We now report a radical-polar crossover (RPC)-enabled three-component cyclization of bromodifluoroalkyls with enaminones and 6-aminouraciles via a visible-light-induced domino cyclization. The reaction exhibited a broad substrate scope (> 40 examples) including complex molecules, which highlighted the utility of this strategy for the construction of a library of bioactive analogs.
Supporting Information
| Filename | Description |
|---|---|
| CJOC202400315-sup-0001-supinfo.pdfPDF document, 6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Nam, G.; Yoon, C. M.; Kim, E.; Rhee, C. K.; Kim, J. H.; Shin, J. H.; Kim, S. H. Syntheses and Evaluation of Pyrido[2,3-d]pyrimidine-2,4- diones as PDE 4 Inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 611–614; (b) Edupuganti, R.; Wang, Q.; Tavares, C. D.; Chitjian, C. A.; Bachman, J. L.; Ren, P.; Anslyn, E. V.; Dalby, K. N. Synthesis and Biological Evaluation of Pyrido[2,3-d]pyrimidine-2,4-dione Derivatives as EEF-2K Inhibitors. Bioorg. Med. Chem. Lett. 2014, 22, 4910−4916; (c) Wang, D. W.; Li, Q.; Wen, K.; Ismail, I.; Liu, D. D.; Niu, C. W.; Wen, X.; Yang, G. F.; Xi, Z. Synthesis and Herbicidal Activity of Pyrido[2,3-d]pyrimidine-2,4-dione-Benzoxazinone Hybrids as Protoporphyrinogen Oxidase Inhibitors. J. Agric. Food Chem. 2017, 65, 5278−5286; (d) Adib, M.; Peytam, F.; Rahmanian-Jazi, M.; Mahernia, S.; Bijanzadeh, H. R.; Jahani, M.; Mohammadi-Khanaposhtani, M.; Imanparast, S.; Faramarzi, M. A.; Mahdavi, M.; Larijani, B. New 6-amino-pyrido[2,3-d]pyrimidine-2,4-diones as Novel Agents to Treat Type 2 Diabetes: A Simple and Efficient Synthesis, α-glucosidase Inhibition, Molecular Modeling and Kinetic Study. Eur. J. Med. Chem. 2018, 155, 353−363; (e) Bulicz, J.; Bertarelli, D. C.; Baumert, D.; Fülle, F.; Müller, C. E.; Heber, D. Synthesis and Pharmacology of Pyrido[2,3-d]pyrimidinediones Bearing Polar Substituents as Adenosine Receptor Antagonists. Bioorg. Med. Chem. Lett. 2006, 14, 2837−2849.
- 2(a) Saikia, P.; Sharma, G.; Gogoi, S.; Boruah, R. C. Cascade Imination, Buchwald–Hartwig Cross Coupling and Cycloaddition Reaction: Synthesis of Pyrido[2,3-d]pyrimidines. RSC Adv. 2015, 5, 23210−23212; (b) Baharfar, R.; Azimi, R. A Clean and Efficient Cyclocondensation to Pyrido[2,3-d]pyrimidine Derivatives in Aqueous Media. Chin. Chem. Lett. 2011, 22, 1183−1186; (c) Cui, X.; Lin, J. J.; Wang, S.; Li, J. P.; Xia, X. S.; Huang, C. Electronic Effect Control of Regioselectivity in the Michael-Addition Inspired Cascade Reaction of 1,3-Dimethyl-6-amino-uracil and 2-Hydroxychalcones. Tetrahedron Lett. 2022, 89, 153603; (d) Castillo, J.; Quiroga, J.; Rodriguez, J.; Coquerel, Y. Time-Efficient Synthesis of Pyrido[2,3-d]pyrimidinones via α-Oxoketenes. Eur. J. Org. Chem. 2016, 2016, 1994−1999.
- 3 Panday, A. K.; Mishra, R.; Jana, A.; Parvin, T.; Choudhury, L. H. Synthesis of Pyrimidine Fused Quinolines by Ligand-Free Copper-Catalyzed Domino Reactions. J. Org. Chem. 2018, 83, 3624−3632.
- 4 Mohammadi Ziarani, G.; Hosseini Nasab, N.; Lashgari, N. Synthesis of Heterocyclic Scaffolds through 6-Aminouracil-involved Multicomponent Reactions. RSC Adv. 2016, 6, 38827−38848.
- 5(a) Rahmati, A.; Khalesi, Z. Catalyst Free Synthesis of Fused Pyrido[2,3-d]pyrimidines and Pyrazolo[3,4-b]pyridines in Water. Chin. Chem. Lett. 2012, 23, 1149–1152; (b) Rahmani, F.; Mohammadpoor- Baltork, I.; Khosropour, A. R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Efficient One-pot Synthesis of New Fused Pyridines and Bis-pyridines Catalyzed by Triazine Diphosphonium Hydrogen Sulfate Ionic Liquid Supported on Functionalized Nano-silica. Tetrahedron Lett. 2016, 57, 2294−2297; (c) Upadhyay, A.; Sharma, L. K.; Singh, V. K.; Singh, R. K. P. An Efficient One Pot Three Component Synthesis of Fused Pyridines via Electrochemical Approach. Tetrahedron Lett. 2016, 57, 5599−5604; (d) Rahmani, F.; Mohammadpoor-Baltork, I.; Khosropour, A. R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Efficient One-pot Synthesis of New Fused Pyridines and Bis-pyridines Catalyzed by Triazine Diphosphonium Hydrogen Sulfate Ionic Liquid Supported on Functionalized Nano-silica. Tetrahedron Lett. 2016, 57, 2294−2297.
- 6(a) Chen, Y.; Lu, L. Q.; Yu, D. G.; Zhu, C. J.; Xiao, W. J. Visible Light- Driven Organic Photochemical Synthesis in China. Sci. China Chem. 2018, 62, 24−57; (b) Ravelli, D.; Protti, S.; Fagnoni, M. Carbon-Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850−9913; (c) Sun, C.; Zhou, Q.; Li, C.-Y.; Hou, Z.-W.; Wang, L. Photoredox-Catalyzed Defluorinative Carboxylation of gem- Difluorostyrenes with Formate Salt. Org. Lett. 2024, 26, 883–888.
- 7(a) Sharma, S.; Singh, J.; Sharma, A. Visible Light Assisted Radical- Polar/Polar-Radical Crossover Reactions in Organic Synthesis. Adv. Synth. Catal. 2021, 363, 3146−3169; (b) Nanda, S. K. Catalytic Radical-Polar Crossover Non-Classical Semipinacol Rearrangements: The Sustainable Approach. Adv. Synth. Catal. 2023, 365, 834−853; (c) Zhu, Z.; Zhang, Y.; Li, Z.; Shu, C. Photoinduced Radical-Polar Crossover Cyclization Reactions. Chem Catal. 2024, 4, 100945; (d) Liu, M.; Ouyang, X.; Xuan, C.; Shu, C. Advances in Photoinduced Radical–Polar Crossover Cyclization (RPCC) of Bifunctional Alkenes. Org. Chem. Front. 2024, 11, 895–915.
- 8(a) Li, H.; Zhang, Y.; Yang, X.; Deng, Z.; Zhu, Z.; Zhou, P.; Ouyang, X.; Yuan, Y.; Chen, X.; Yang, L.; Liu, M.; Shu, C. Synthesis of Multifluoromethylatedγ-Sultines by a Photoinduced Radical Addition-Polar Cyclization. Angew. Chem. Int. Ed. 2023, 62, e202300159; (b) Dhungana, R. K.; Granados, A.; Ciccone, V.; Martin, R. T.; Majhi, J.; Sharique, M.; Gutierrez, O.; Molander, G. A. Trifunctionalization of Cinnamyl Alcohols Provides Access to Brominated α,α-Difluoro-γ-lactones via a Photoinduced Radical-Polar-Radical Mechanism. ACS Catal. 2022, 12, 15750−15757; (c) Zhao, P.; Wang, L.; Guo, X.; Chen, J.; Liu, Y.; Wang, L.; Ma, Y. Visible Light-Driven α-Diazoketones as Denitrogenated Synthons: Synthesis of Fluorinated N-Heterocycles via Multicomponent Cyclization Reactions. Org. Lett. 2023, 25, 3314−3318; (d) Zhang, F.; Liao, S.; Zhou, L.; Yang, K.; Wang, C.; Lou, Y.; Wang, C.; Song, Q. An Olefinic 1,2-α-Boryl Migration Enables 1,2-Bis(boronic esters) via Radical-Polar Crossover Reaction. Chin. J. Chem. 2022, 40, 582−588; (e) Zuo, W.; Zuo, L.; Geng, X.; Li, Z.; Wang, L. Photoinduced C–H Heteroarylation of Enamines via Quadruple Cleavage of CF2Br2. Org. Chem. Front. 2023, 10, 6112−6116; (f) Zuo, W.; Zuo, L.; Geng, X.; Li, Z.; Wang, L. Radical-Polar Crossover Enabled Triple Cleavage of CF2Br2: A Multicomponent Tandem Cyclization to 3-Fluoropyrazoles. Org. Lett. 2023, 25, 6062–6066. (g) Zhao, P.; Liu, Y.; Zhang, Y.; Wang, L.; Ma, Y. Photodriven Radical-Polar Crossover Cyclization Strategy: Synthesis of Pyrazolo[1,5-a]pyridines from Diazo Compounds. Org. Lett. 2024, 26, 2511−2516.
- 9 Ma, X.; Song, Q. Recent Progress on Selective Deconstructive Modes of Halodifluoromethyl and Trifluoromethyl-Containing Reagents. Chem. Soc. Rev. 2020, 49, 9197−9219.
- 10(a) Deng, S.; Chen, H.; Ma, X.; Zhou, Y.; Yang, K.; Lan, Y.; Song, Q. S8-Catalyzed Triple Cleavage of Bromodifluoro Compounds for the Assembly of N-containing Heterocycles. Chem. Sci. 2019, 10, 6828−6833; (b) Ma, X.; Yu, X.; Huang, H.; Zhou, Y.; Song, Q. Synthesis of Thiazoles and Isothiazoles via Three-Component Reaction of Enaminoesters, Sulfur, and Bromodifluoroacetamides/Esters. Org. Lett. 2020, 22, 5284−5288.
- 11 Liu, Y.; Luo, W.; Wang, Z.; Zhao, Y.; Zhao, J.; Xu, X.; Wang, C.; Li, P. Visible-Light Photoredox-Catalyzed Formal [5 + 1] Cycloaddition of N-Tosyl Vinylaziridines with Difluoroalkyl Halides. Org. Lett. 2020, 22, 9658−9664.
- 12(a) Geng, X.; Xu, Z.; Cai, Y.; Wang, L. Visible-Light-Driven Multicomponent Cyclization by Trapping a 1,3-Vinylimine Ion Intermediate: A Direct Approach to Pyrimido[1,2-b]indazole Derivatives. Org. Lett. 2021, 23, 8343−8347; (b) Huo, J.; Geng, X.; Li, W.; Zhang, P.; Wang, L. A Traceless Heterocyclic Amine Mediator in Regioselectivity− Switchable Formal [1 + 2 + 2] Cycloaddition Reaction to 1,3,4- and 1,4,5-Trisubstituted Pyrazoles. Org. Lett. 2023, 25, 512−516.
- 13(a) Ali, D.; Panday, A. K.; Choudhury, L. H. Hydrogen Peroxide-Mediated Rapid Room Temperature Metal-Free C(sp2)-H Thiocyanation of Amino Pyrazoles, Amino Uracils, and Enamines. J. Org. Chem. 2020, 85, 13610−13620; (b) Ali, D.; Parvin, T.; Choudhury, L. H. Visible Light-Mediated C(sp2)–H Selenylation of Amino Pyrazole and Amino Uracils in the Presence of Rose Bengal as an Organophotocatalyst. J. Org. Chem. 2022, 87, 1230−1239.
- 14(a) Zhuang, X.; Ling, L.; Wang, Y.; Li, B.; Sun, B.; Su, W.; Jin, C. Photoinduced Cascade C−N/C=O Bond Formation from Bromodifluoroalkyl Reagents, Amines, and H2O via a Triple-Cleavage Process. Org. Lett. 2022, 24, 1668−1672;
(b) Dhungana, R. K.; Granados, A.; Sharique, M.; Majhi, J.; Molander, G. A. A Three-component Difunctionalization of N-Alkenyl Amides via Organophotoredox Radical- polar Crossover. Chem. Commun. 2022, 58, 9556−9559;
(c) Hou, Z. W.; Xu, H. C.; Wang, L. Electrochemical Generation and Utilization of Radical Intermediates. Curr. Opin. Electrochem. 2024, 44, 101447;
(d) He, H.; Lv, Y.; Hu, J.; Hou. Z.-W.; Wang, L. Seminormal-BrCH2CH2OH- Mediated Electrochemical Epoxidation of Unactivated Olefins. Green Chem. 2024, 26, 2157–2161;
(e) Luo, Y.; Dong, L. Photo-Mediated para-Selective C(sp2)−H Difluoroalkylations. Green Synth. Catal. 2023, DOI: https://doi.org/10.1016/j.gresc.2023.11.005;
10.1016/j.gresc.2023.11.005 Google Scholar(f) Zhang, M.; Li, Q.; Lin, J. H.; Xiao, J. C. Difluoroalkylation/Lactonization of Alkenes with BrCF2CO2K via Photoredox Catalysis: Access to α,α-Difluoro-γ-lactones. Chin. J. Chem. 2023, 41, 2819−2824; (g) Yang, J. X.; Hu, J. Q.; Wen, Q.; Xu, J.; Zhang, Y.; Jiang, H. Photoexcitation of Dihydroquinazolinone Anionic Compounds: Difluoroalkylarylation and Difluoroalkylamination of Alkenes. CCS Chem. 2024, DOI: https://doi.org/10.31635/ccschem.024.202303480.10.31635/ccschem.024.202303480 Google Scholar
- 15 Han, Y.; Zhou, L.; Wang, C.; Feng, S.; Ma, R.; Wan, J. P. Recent Advances in Visible Light-mediated Chemical Transformations of Enaminones. Chin. Chem. Lett. 2024, 35, 108977.




