Co/Fe Dual Catalysis for Sequential Hydrosilylation–Isomerization: Access to Trisubstituted (E)-Alkenyl Silanes from Terminal Alkynes
Zhihao Guo
Chang-Kung Chuang Institute, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorGuixia Liu
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Zheng Huang
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute of Advanced Study, University of Chinese Academy of Sciences, Hangzhou, Zhejiang, 310024 China
E-mail: [email protected]Search for more papers by this authorZhihao Guo
Chang-Kung Chuang Institute, East China Normal University, Shanghai, 200062 China
Search for more papers by this authorGuixia Liu
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Zheng Huang
The State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200032 China
School of Chemistry and Materials Science, Hangzhou Institute of Advanced Study, University of Chinese Academy of Sciences, Hangzhou, Zhejiang, 310024 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
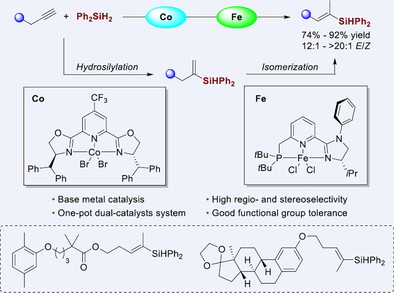
By rational modification of electronic and steric properties of pincer ligands, a Co/Fe dual catalyst system is developed for one-pot sequential Markovnikov alkyne hydrosilylation and stereoselective alkene isomerization. The protocol provides an atom-economical and efficient approach to trisubstituted (E)-alkenyl silanes from widely accessible terminal alkynes with high regio- and stereoselectivities under mild conditions. The utility of this reaction was demonstrated by gram-scale synthesis and derivatization of bioactive molecules. The radical clock and trapping experiments indicated that radical pathway might be operative in the alkene isomerization step.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400291-sup-0001-Supinfo.pdfPDF document, 7.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Fleming, I.; Barbero, A.; Walter, D. Stereochemical Control in Organic Synthesis Using Silicon-Containing Compounds. Chem. Rev. 1997, 97, 2063–2192; (b) Curtis-Long, M. J.; Aye, Y. Vinyl-, Propargyl-, and Allenylsilicon Reagents in Asymmetric Synthesis: A Relatively Untapped Resource of Environmentally Benign Reagents. Chem. Eur. J. 2009, 15, 5402–5416; (c) Nakao, Y.; Hiyama, T. Silicon-based cross-coupling reaction: an environmentally benign version. Chem. Soc. Rev. 2011, 40, 4893–4901; (d) Sore, H. F.; Galloway, W. R. J. D.; Spring, D. R. Palladium-catalysed cross-coupling of organosilicon reagents. Chem. Soc. Rev. 2012, 41, 1845–1866; (e) Szudkowska-Frątczak, J.; Hreczycho, G.; Pawluć, P. Silylative coupling of olefins with vinylsilanes in the synthesis of functionalized alkenes. Org. Chem. Front. 2015, 2, 730–738; (f) Komiyama, T.; Minami, Y.; Hiyama, T. Recent Advances in Transition-Metal-Catalyzed Synthetic Transformations of Organosilicon Reagents. ACS Catal. 2017, 7, 631–651; (g) Marciniec, B. Silicometallics vs. Organometallics and Catalysis: General Guidelines. In Perspectives of Hydrosilylation Reactions, Eds.: Marciniec, B.; Maciejewski, H., Springer Nature Switzerland, Cham, 2023, pp. 1–12.
- 2(a) Itami, K.; Yoshida, J.-i. Multisubstituted Olefins: Platform Synthesis and Applications to Materials Science and Pharmaceutical Chemistry. Bull. Chem. Soc. Jpn. 2006, 79, 811–824; (b) Negishi, E.-i.; Huang, Z.; Wang, G.; Mohan, S.; Wang, C.; Hattori, H. Recent Advances in Efficient and Selective Synthesis of Di-, Tri-, and Tetrasubstituted Alkenes via Pd-Catalyzed Alkenylation−Carbonyl Olefination Synergy. Acc. Chem. Res. 2008, 41, 1474–1485; (c) Denmark, S. E.; Liu, J. H.-C. Silicon-Based Cross-Coupling Reactions in the Total Synthesis of Natural Products. Angew. Chem. Int. Ed. 2010, 49, 2978–2986; (d) Bergueiro, J.; Montenegro, J.; Cambeiro, F.; Saá, C.; López, S. Cross-Coupling Reactions of Organosilicon Compounds in the Stereocontrolled Synthesis of Retinoids. Chem. Eur. J. 2012, 18, 4401–4410; (e) Urabe, D.; Asaba, T.; Inoue, M. Convergent Strategies in Total Syntheses of Complex Terpenoids. Chem. Rev. 2015, 115, 9207–9231; (f) Korch, K. M.; Watson, D. A. Cross-Coupling of Heteroatomic Electrophiles. Chem. Rev. 2019, 119, 8192–8228.
- 3(a) Fleming, I.; Newton, T. W.; Roessler, F. The silylcupration of acetylenes: a synthesis of vinylsilanes. J. Chem. Soc., Perkin Trans. 1 1981, 2527–2532;
(b) Barbero, A.; Cuadrado, P.; Fleming, I.; González, A. M.; Pulido, F. J.; Sánchez, A. Synthesis of vinylsilanes by silyl-cupration of acetylenes using tert-butyldiphenylsilyl-cuprate reagents. J. Chem. Soc., Perkin Trans. 1 1995, 1525–1532;
10.1039/P19950001525 Google Scholar(c) Coleman, R. S.; Walczak, M. C.; Campbell, E. L. Total Synthesis of Lucilactaene, a Cell Cycle Inhibitor Active in p53-Inactive Cells. J. Am. Chem. Soc. 2005, 127, 16038–16039; (d) Fuwa, H.; Ebine, M.; Sasaki, M. Total Synthesis of the Proposed Structure of Brevenal. J. Am. Chem. Soc. 2006, 128, 9648–9650; (e) Valot, G.; Regens, C. S.; O'Malley, D. P.; Godineau, E.; Takikawa, H.; Fürstner, A. Total Synthesis of Amphidinolide F. Angew. Chem. Int. Ed. 2013, 52, 9534–9538; (f) Preindl, J.; Schulthoff, S.; Wirtz, C.; Lingnau, J.; Fürstner, A. Polyunsaturated C-Glycosidic 4-Hydroxy-2-pyrone Derivatives: Total Synthesis Shows that Putative Orevactaene Is Likely Identical with Epipyrone A. Angew. Chem. Int. Ed. 2017, 56, 7525–7530; (g) Anderl, F.; Größl, S.; Wirtz, C.; Fürstner, A. Total Synthesis of Belizentrin Methyl Ester: Report on a Likely Conquest. Angew. Chem. Int. Ed. 2018, 57, 10712–10717; (h) Löffler, L. E.; Wirtz, C.; Fürstner, A. Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets. Angew. Chem. Int. Ed. 2021, 60, 5316–5322.
- 4(a) Oestreich, M.; Hartmann, E.; Mewald, M. Activation of the Si–B Interelement Bond: Mechanism, Catalysis, and Synthesis. Chem. Rev. 2013, 113, 402–441; (b) Wilkinson, J. R.; Nuyen, C. E.; Carpenter, T. S.; Harruff, S. R.; Van Hoveln, R. Copper-Catalyzed Carbon–Silicon Bond Formation. ACS Catal. 2019, 9, 8961–8979; (c) Feng, J.-J.; Mao, W.; Zhang, L.; Oestreich, M. Activation of the Si–B interelement bond related to catalysis. Chem. Soc. Rev. 2021, 50, 2010–2073; (d) Marciniec, B.; Pietraszuk, C.; Pawluć, P.; Maciejewski, H. Inorganometallics (Transition Metal–Metalloid Complexes) and Catalysis. Chem. Rev. 2022, 122, 3996–4090.
- 5(a) Marciniec, B. Hydrosilylation of Alkynes and Their Derivatives. In Hydrosilylation: A Comprehensive Review on Recent Advances, Ed.: Marciniec, B., Springer Netherlands, Dordrecht, 2009, pp. 53–86; (b) Zaranek, M.; Marciniec, B.; Pawluć, P. Ruthenium-catalysed hydrosilylation of carbon–carbon multiple bonds. Org. Chem. Front. 2016, 3, 1337–1344; (c) Chen, J.; Guo, J.; Lu, Z. Recent Advances in Hydrometallation of Alkenes and Alkynes via the First Row Transition Metal Catalysis. Chin. J. Chem. 2018, 36, 1075–1109; (d) Wen, H.; Liu, G.; Huang, Z. Recent advances in tridentate iron and cobalt complexes for alkene and alkyne hydrofunctionalizations. Coord. Chem. Rev. 2019, 386, 138–153; (e) Chen, W.; Li, J.; Cui, C. Rare-Earth- Catalyzed Hydrosilylation and Dehydrogenative Coupling of Hydrosilanes. Synlett 2020, 32, 962–970; (f) de Almeida, L. D.; Wang, H.; Junge, K.; Cui, X.; Beller, M. Recent Advances in Catalytic Hydrosilylations: Developments Beyond Traditional Platinum Catalysts. Angew. Chem. Int. Ed. 2021, 60, 550–565; (g) He, P.; Hu, M.-Y.; Zhang, X.-Y.; Zhu, S.-F. Transition-Metal-Catalyzed Stereo- and Regioselective Hydrosilylation of Unsymmetrical Alkynes. Synthesis 2021, 54, 49–66.
- 6 Molander, G. A.; Retsch, W. H. Selective Hydrosilylation of Alkynes Catalyzed by an Organoyttrium Complex. Organometallics 1995, 14, 4570–4575.
- 7 Trost, B. M.; Ball, Z. T. Alkyne Hydrosilylation Catalyzed by a Cationic Ruthenium Complex: Efficient and General Trans Addition. J. Am. Chem. Soc. 2005, 127, 17644–17655.
- 8 Chaulagain, M. R.; Mahandru, G. M.; Montgomery, J. Alkyne hydrosilylation catalyzed by nickel complexes of N-heterocyclic carbenes. Tetrahedron 2006, 62, 7560–7566.
- 9 Yong, L.; Kirleis, K.; Butenschön, H. Stereodivergent Formation of Alkenylsilanes: syn or anti Hydrosilylation of Alkynes Catalyzed by a Cyclopentadienylcobalt(I) Chelate Bearing a Pendant Phosphane Tether. Adv. Synth. Catal. 2006, 348, 833–836.
- 10 Berthon-Gelloz, G.; Schumers, J.-M.; De Bo, G.; Markó, I. E. Highly β-(E)-Selective Hydrosilylation of Terminal and Internal Alkynes Catalyzed by a (IPr)Pt(diene) Complex. J. Org. Chem. 2008, 73, 4190–4197.
- 11 Guo, J.; Lu, Z. Highly Chemo-, Regio-, and Stereoselective Cobalt-Catalyzed Markovnikov Hydrosilylation of Alkynes. Angew. Chem. Int. Ed. 2016, 55, 10835–10838.
- 12 Wu, G.; Chakraborty, U.; Jacobi von Wangelin, A. Regiocontrol in the cobalt-catalyzed hydrosilylation of alkynes. Chem. Commun. 2018, 54, 12322–12325.
- 13 Hu, M.-Y.; He, P.; Qiao, T.-Z.; Sun, W.; Li, W.-T.; Lian, J.; Li, J.-H.; Zhu, S.-F. Iron-Catalyzed Regiodivergent Alkyne Hydrosilylation. J. Am. Chem. Soc. 2020, 142, 16894–16902.
- 14 Li, H.; Yang, C.; Wang, D.; Deng, L. Cobalt-Catalyzed Regio- and Stereoselective Hydrosilylation of Alk-2-ynes with Tertiary Silanes. Organometallics 2023, 42, 1693–1698.
- 15(a) Uma, R.; Crévisy, C.; Grée, R. Transposition of Allylic Alcohols into Carbonyl Compounds Mediated by Transition Metal Complexes. Chem. Rev. 2003, 103, 27–52; (b) Krompiec, S.; Krompiec, M.; Penczek, R.; Ignasiak, H. Double bond migration in N-allylic systems catalyzed by transition metal complexes. Coord. Chem. Rev. 2008, 252, 1819–1841; (c) Hassam, M.; Taher, A.; Arnott, G. E.; Green, I. R.; van Otterlo, W. A. L. Isomerization of Allylbenzenes. Chem. Rev. 2015, 115, 5462–5569; (d) Vasseur, A.; Bruffaerts, J.; Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 2016, 8, 209–219; (e) Liu, X.; Li, B.; Liu, Q. Base-Metal-Catalyzed Olefin Isomerization Reactions. Synthesis 2019, 51, 1293–1310.
- 16(a) Stille, J. K.; Becker, Y. Isomerization of N-allylamides and -imides to aliphatic enamides by iron, rhodium, and ruthenium complexes. J. Org. Chem. 1980, 45, 2139–2145; (b) Grotjahn, D. B.; Larsen, C. R.; Gustafson, J. L.; Nair, R.; Sharma, A. Extensive Isomerization of Alkenes Using a Bifunctional Catalyst: An Alkene Zipper. J. Am. Chem. Soc. 2007, 129, 9592–9593; (c) Crossley, S. W. M.; Barabé, F.; Shenvi, R. A. Simple, Chemoselective, Catalytic Olefin Isomerization. J. Am. Chem. Soc. 2014, 136, 16788–16791; (d) Li, G.; Kuo, J. L.; Han, A.; Abuyuan, J. M.; Young, L. C.; Norton, J. R.; Palmer, J. H. Radical Isomerization and Cycloisomerization Initiated by H• Transfer. J. Am. Chem. Soc. 2016, 138, 7698–7704; (e) Huang, R.-Z.; Lau, K. K.; Li, Z.; Liu, T.-L.; Zhao, Y. Rhodium-Catalyzed Enantioconvergent Isomerization of Homoallylic and Bishomoallylic Secondary Alcohols. J. Am. Chem. Soc. 2018, 140, 14647–14654.
- 17(a) Zhang, S.; Bedi, D.; Cheng, L.; Unruh, D. K.; Li, G.; Findlater, M. Cobalt(II)-Catalyzed Stereoselective Olefin Isomerization: Facile Access to Acyclic Trisubstituted Alkenes. J. Am. Chem. Soc. 2020, 142, 8910–8917; (b) Zhao, J.; Cheng, B.; Chen, C.; Lu, Z. Cobalt-Catalyzed Migrational Isomerization of Styrenes. Org. Lett. 2020, 22, 837–841; (c) Liu, H.; Cai, C.; Ding, Y.; Chen, J.; Liu, B.; Xia, Y. Cobalt-Catalyzed E-Selective Isomerization of Alkenes with a Phosphine-Amido-Oxazoline Ligand. ACS Omega 2020, 5, 11655–11670; (d) Yu, X.; Zhao, H.; Li, P.; Koh, M. J. Iron-Catalyzed Tunable and Site-Selective Olefin Transposition. J. Am. Chem. Soc. 2020, 142, 18223–18230; (e) Hu, X.-S.; He, J.-X.; Zhang, Y.; Zhou, J.; Yu, J.-S. Highly Stereoselective Positional Isomerization of Styrenes via Acid-Catalyzed Carbocation Mechanism. Chin. J. Chem. 2021, 39, 2227–2233.
- 18(a) Liu, X.; Zhang, W.; Wang, Y.; Zhang, Z.-X.; Jiao, L.; Liu, Q. Cobalt-Catalyzed Regioselective Olefin Isomerization Under Kinetic Control. J. Am. Chem. Soc. 2018, 140, 6873–6882; (b) Kapat, A.; Sperger, T.; Guven, S.; Schoenebeck, F. E-Olefins through intramolecular radical relocation. Science 2019, 363, 391–396; (c) Liu, X.; Rong, X.; Liu, S.; Lan, Y.; Liu, Q. Cobalt-Catalyzed Desymmetric Isomerization of Exocyclic Olefins. J. Am. Chem. Soc. 2021, 143, 20633–20639; (d) Rong, X.; Yang, J.; Liu, S.; Lan, Y.; Liu, Q. Remote Stereocontrol of All-Carbon Quaternary Centers via Cobalt-Catalyzed Asymmetric Olefin Isomerization. CCS Chem. 2023, 5, 1293–1300.
- 19 Xu, S.; Geng, P.; Li, Y.; Liu, G.; Zhang, L.; Guo, Y.; Huang, Z. Pincer Iron Hydride Complexes for Alkene Isomerization: Catalytic Approach to Trisubstituted (Z)-Alkenyl Boronates. ACS Catal. 2021, 11, 10138–10147.
- 20 Shimada, S. Recent Advances of Group 10 Transition Metal Hydrosilylation Catalysts. In Perspectives of Hydrosilylation Reactions, Eds.: Marciniec, B.; Maciejewski, H., Springer Nature Switzerland, Cham, 2023, pp. 13–93.
- 21(a) Zuo, Z.; Yang, J.; Huang, Z. Cobalt-Catalyzed Alkyne Hydrosilylation and Sequential Vinylsilane Hydroboration with Markovnikov Selectivity. Angew. Chem. Int. Ed. 2016, 55, 10839–10843; (b) Wen, H.; Wan, X.; Huang, Z. Asymmetric Synthesis of Silicon-Stereogenic Vinylhydrosilanes by Cobalt-Catalyzed Regio- and Enantioselective Alkyne Hydrosilylation with Dihydrosilanes. Angew. Chem. Int. Ed. 2018, 57, 6319–6323; (c) Skrodzki, M.; Patroniak, V.; Pawluć, P. Schiff Base Cobalt(II) Complex-Catalyzed Highly Markovnikov-Selective Hydrosilylation of Alkynes. Org. Lett. 2021, 23, 663–667; (d) Wang, D.; Lai, Y.; Wang, P.; Leng, X.; Xiao, J.; Deng, L. Markovnikov Hydrosilylation of Alkynes with Tertiary Silanes Catalyzed by Dinuclear Cobalt Carbonyl Complexes with NHC Ligation. J. Am. Chem. Soc. 2021, 143, 12847–12856; (e) Sahoo, M. K.; Kim, D.; Chang, S.; Park, J.-W. Regioselective Access to α-Vinylsilanes and α-Vinylgermanes by Cobalt-Catalyzed Migratory Hydrofunctionalization of 2-Alkynes. ACS Catal. 2021, 11, 12777–12784; (f) Skrodzki, M.; Ortega Garrido, V.; Csáky, A. G.; Pawluć, P. Searching for highly active cobalt catalysts bearing Schiff base ligands for Markovnikov-selective hydrosilylation of alkynes with tertiary silanes. J. Catal. 2022, 411, 116–121; (g) Bołt, M.; Żak, P. Bulky NHC–Cobalt Complex-Catalyzed Highly Markovnikov-Selective Hydrosilylation of Alkynes. Catalysts 2023, 13, 510.
- 22 Mayer, M.; Welther, A.; Jacobi von Wangelin, A. Iron-Catalyzed Isomerizations of Olefins. ChemCatChem 2011, 3, 1567–1571.
- 23 Xu, S.; Liu, G.; Huang, Z. Iron Catalyzed Isomerization of α-Alkyl Styrenes to Access Trisubstituted Alkenes. Chin. J. Chem. 2021, 39, 585–589.
- 24 1s could be hydrosilylated by Co-1g to generate 3s in 83% yield without any ring-opening products. Catalytic amounts of Co-1g and Fe-3 were independently employed in isomerization of 3s at 60 °C for 18 h. The reaction catalyzed by Fe-3 delivered the ring opening product (3s’) in 16% NMR yield with 75% of 3s recovered, whereas no reaction occurred with Co-1g as the catalyst.
- 25(a) Chen, C.; Shen, X.; Chen, J.; Hong, X.; Lu, Z. Iron-Catalyzed Hydroboration of Vinylcyclopropanes. Org. Lett. 2017, 19, 5422–5425; (b) Chen, C.; Wang, H.; Li, T.; Lu, D.; Li, J.; Zhang, X.; Hong, X.; Lu, Z. Cobalt-Catalyzed Asymmetric Sequential Hydroboration/Isomerization/Hydroboration of 2-Aryl Vinylcyclopropanes. Angew. Chem. Int. Ed. 2022, 61, e202205619.




