Hyperlanins A and B, Two Highly Rearranged Meroterpenoids from Hypericum lancasteri
Jin-Qiu You
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorYun-Qi Jin
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorMeng-Yu Li
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorBo-Chao Zhang
Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China
Search for more papers by this authorXin-Yu Sun
Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorChun-Yong Ding
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorJing-Ya Li
Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Ai-Jun Hou
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]Search for more papers by this authorJin-Qiu You
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorYun-Qi Jin
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorMeng-Yu Li
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorBo-Chao Zhang
Shanghai Jiao Tong University School of Medicine, Shanghai, 200025 China
Search for more papers by this authorXin-Yu Sun
Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorChun-Yong Ding
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorJing-Ya Li
Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203 China
Search for more papers by this authorCorresponding Author
Ai-Jun Hou
Shanghai Frontiers Science Center of Drug Target Identification and Delivery, School of Pharmaceutical Sciences, Shanghai Jiao Tong University, Shanghai, 200240 China
National Key Laboratory of Innovative Immunotherapy, Shanghai Jiao Tong University, Shanghai, 200240 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
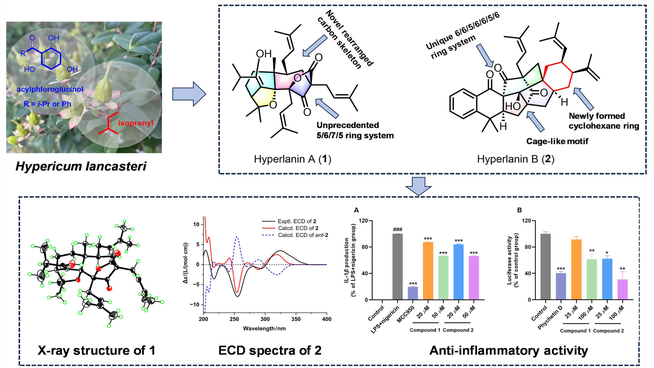
Hyperlanins A (1) and B (2), two highly rearranged polycyclic polyprenylated acylphloroglucinol (PPAP)-related meroterpenoids based on different new carbon skeletons, were isolated from Hypericum lancasteri. Compound 1 incorporates an unprecedented 5/6/7/5 ring system featuring a 3,13-dioxatetracyclo[9.2.1.12,5.01,8]pentadecane core. Compound 2 possesses a unique compact 6/6/5/6/6/5/6 ring system with a caged tetracyclo[6.2.1.13,8.05,11]dodecane motif. Their structures were established by spectroscopic data, X-ray diffraction, and computational approaches. Both compounds showed anti-inflammatory activity in vitro. Compounds 1 and 2 could decrease the lipopolysaccharide (LPS)-/nigericin-induced IL-1β release in THP-1 cells. Both compounds also showed inhibition in hypoxia-inducible factor-1α (HIF-1α) pathway luciferase reporter assay.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400248-sup-0001-supinfo.pdfPDF document, 4.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Flora of China Editorial Committee. In Flora Reipublicae Popularis Sinicae, Science Press, Beijing, 1990, Vol. 50, p. 33.
- 2 Caldeira, G. I.; Gouveia, L. P.; Serrano, R.; Silva, O. D. Hypericum genus as a natural source for biologically active compounds. Plants 2022, 11, 2509.
- 3 Marrelli, M.; Statti, G.; Conforti, F. Hypericum spp.: an update on the biological bctivities and metabolic profiles. Mini-Rev. Med. Chem. 2020, 20, 66−87.
- 4 Bridi, H.; Meirelles, G. C.; von Poser, G. L. Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 2018, 155, 203−232.
- 5 Galeotti, N. Hypericum perforatum (St John's wort) beyond depression: a therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136−146.
- 6 Marrelli, M.; Statti, G.; Conforti F.; Menichini, F. New potential pharmaceutical applications of Hypericum species. Mini-Rev. Med. Chem. 2016, 16, 710−720.
- 7 Ciochina, R.; Grossman, R. B. Polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2006, 106, 3963−3986.
- 8 Yang, X. W.; Grossman, R. B.; Xu, G. Research progress of polycyclic polyprenylated acylphloroglucinols. Chem. Rev. 2018, 118, 3508−3558.
- 9 Yang, B.; Su, J. C.; Huang, L.; Lin, S.; Jin, X.; Lei, X.; Hu, Z.; Zhang, Y. Hyperispirones A and B, spiro-bridged polycyclic polyprenylated acylphloroglucinols with anti-angiogenesis activity from Hypericum beanie. Org. Chem. Front. 2022, 9, 3460−3466.
- 10 Lu, W. J.; Xu, W. J.; Zhang, Y. Q.; Li, Y. R.; Zhou, X.; Li, Q. J.; Zhang, H.; Luo, J.; Kong, L. Y. Hyperforones A–C, benzoyl-migrated [5.3.1]-type polycyclic polyprenylated acylphloroglucinols from Hypericum forrestii. Org. Chem. Front. 2020, 7, 1070−1076.
- 11 Zhang, J. J.; Yang, J.; Liao, Y.; Yang, X. W.; Ma, J. Z.; Xiao, Q. L.; Yang, L. X.; Xu, G. Hyperuralones A and B, new acylphloroglucinol derivatives with intricately caged cores from Hypericum uralum. Org. Lett. 2014, 16, 4912−4915.
- 12 Richard, J. A.; Pouwer, R. H.; Chen, D. Y. K. The chemistry of the polycyclic polyprenylated acylphloroglucinols. Angew. Chem. 2012, 51, 4536−4561.
- 13 Grossman, R. B. Table of PPAPs, http://www.uky.edu/~rbgros1/ PPAPs/http://www.uky.edu/~rbgros1/PPAPs/ (accessed March 1, 2024).
- 14 Tanaka, N.; Kashiwada, Y. Characteristic metabolites of Hypericum plants: their chemical structures and biological activities. J. Nat. Med. 2021, 75, 423−433.
- 15 Guttroff, C.; Baykal, A.; Wang, H.; Popella, P.; Kraus, F.; Biber, N.; Krauss, S.; Götz, F.; Plietker, B. Polycyclic polyprenylated acylphloroglucinols: an emerging class of non-peptide-based MRSA- and VRE-active antibiotics. Angew. Chem. Int. Ed. 2017, 56, 15852−15856.
- 16 Jia, X.; Wu, Y.; Lei, C.; Yu, Y.; Li, J.; Li, J.; Hou, A. Hyperinoids A and B, two polycyclic meroterpenoids from Hypericum patulum. Chin. Chem. Lett. 2020, 31, 1263−1266.
- 17 Zong, J. F.; Hu, Z.; Shao, Y. Y.; Shi, Q.; Zhang, M. M.; Zhou, Y. B.; Li, J.; Hou, A. J. Hyperprins A and B, two complex meroterpenoids from Hypericum przewalskii. Org. Lett. 2020, 22, 2797−2800.
- 18 Huang, J. C.; Sheng, L.; Zong, J. F.; Zhou, Y. B.; Li, J.; Hou, A. J. Enantiomeric pairs of meroterpenoids with 11/5/6 spiro-heterocyclic systems from Hypericum kouytchense. Org. Chem. Front. 2022, 9, 6475−6483.
- 19 Hashida, C.; Tanaka, N.; Kawazoe, K.; Murakami, K.; Sun, H. D.; Takaishi, Y.; Kashiwada, Y. Hypelodins A and B, polyprenylated benzophenones from Hypericum elodeoides. J. Nat. Med. 2014, 68, 737–742.
- 20 Lodewyk, M. W.; Siebert, M. R.; Tantillo, D. J. Computational prediction of 1H and 13C chemical shifts: a useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839−1862.
- 21 Gao, W.; Hou, W. Z.; Zhao, J.; Xu, F.; Li, L.; Xu, F.; Sun, H.; Xing, J. G.; Peng, Y.; Wang, X. L.; Ji, T. F.; Gu, Z. Y. Polycyclic polyprenylated acylphloroglucinol congeners from Hypericum scabrum. J. Nat. Prod. 2016, 79, 1538−1547.
- 22 Yang, X. W.; Ding, Y.; Zhang, J. J.; Liu, X.; Yang, L. X.; Li, X. N.; Ferreira, D.; Walker, L. A.; Xu, G. New acylphloroglucinol derivatives with diverse architectures from Hypericum henryi. Org. Lett. 2014, 16, 2434−2437.
- 23 Grivennikov, S. I.; Greten, F. R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883−899.
- 24 Sporn, M. B.; Liby, K. T.; Yore, M. M.; Fu, L.; Lopchuk, J. M.; Gribble, G. W. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J. Nat. Prod. 2011, 74, 537−545.
- 25 You, J. Q.; Liu, Y. N.; Zhou, J. S.; Sun, X. Y.; Lei, C.; Mu, Q.; Li, J. Y.; Hou, A. J. cis-Clerodane diterpenoids with structural diversity and anti-inflammatory activity from Tinospora crispa. Chin. J. Chem. 2022, 40, 2882−2892.
- 26 Li, Y. T.; Li, S. F.; Lei, C.; You, J. Q.; Huang, J. C. Hou, A. J. Dimeric sesquiterpenoids and anti-inflammatory constituents of Sarcandra glabra. Bioorg. Chem. 2022, 124, 105821.
- 27 Shi, Q.; Li, T. T.; Wu, Y. M.; Sun, X. Y.; Lei, C.; Li, J. Y.; Hou, A. J. Meroterpenoids with diverse structures and anti-inflammatory activities from Rhododendron anthopogonoides. Phytochemistry 2020, 180, 112524.
- 28 Perera, A. P.; Fernando, R.; Shinde, T.; Gundamaraju, R.; Southam, B.; Sohal, S. S.; Robertson, A. A. B.; Schroder, K.; Kunde, D.; Eri, R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018, 8, 8618.
- 29 Corrado, C.; Fontana, S. Hypoxia and HIF signaling: one axis with divergent effects. Int. J. Mol. Sci. 2020, 21, 5611.
- 30 Ji, K.; Liu, W.; Yin, W.; Kong, X.; Xu, H.; Lai, Z. W.; Li, J. Y.; Yue, J. M. A new class of potent liver injury protective compounds: structural elucidation, total synthesis and bioactivity study. Acta Pharm. Sin. B 2023, 13, 3414–3424.




