Revealing the Regulation Effect of Surface Charge at Aromatic Interface to Dynamic Conformational Changes of α-Synuclein at Early Aggregation Stage
Yuqi Zhang
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Lie Wu
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiue Jiang
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYuqi Zhang
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Lie Wu
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiue Jiang
State Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin, 130022 China
School of Applied Chemistry and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Research Center for Analytical Science, College of Chemistry, Nankai University, Tianjin, 300071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
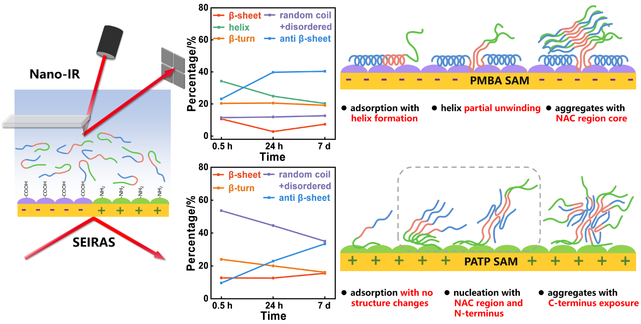
The aggregation of α-synuclein (α-syn) is strongly influenced by membrane interfaces, but the mechanism of transition from monomers to oligomers at early aggregation stage is not clear. Here, we investigate the adsorption and structure changes of α-syn on oppositely charged aromatic interfaces through in-situ surface-enhanced infrared absorption (SEIRA) spectroscopy and nano-IR technique. The results show that the synergy of electrostatic and hydrophobic interactions leads to a “fast-slow” two-step aggregation pathway on negatively charged interface. Surface adsorption induces the formation of an extended helix structure and subsequently partial helix unwinding in NAC region, which enables the hydrophobic stacking between nearby NAC regions. Stable antiparallel β-sheet rich aggregates are gradually emerging as further interactions of monomers with the fast formed “first layer”. Monomers electrostatically adsorb on positively charged interface by C-terminus with NAC region and N-terminus stretched in solvent, which serve as an aggregation core and induce further adsorption and gradual formation of aggregates with C-terminus exposure. Our results demonstrate the modulation of surface charge and synergy of electrostatic and hydrophobic interactions on the interaction modes and aggregation pathways, which provide insights into dynamic conformation changes of α-syn at early aggregation stage and imply the important role of spatial-temporal heterogeneity of membranes in α-synucleinopathies.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400155_sup_0001_supinfo.pdfPDF document, 633 KB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Strohäker, T.; Jung, B. C.; Liou, S. H.; Fernandez, C. O.; Riedel, D.; Becker, S.; Halliday, G. M.; Bennati, M.; Kim, W. S.; Lee, S. J.; et al. Structural Heterogeneity of α-Synuclein Fibrils Amplified from Patient Brain Extracts. Nat. Commun. 2019, 10, 5535; (b) Goldstein, D. S.; Isonaka, R.; Lamotte, G.; Kaufmann, H. Different Phenoconversion Pathways in Pure Autonomic Failure with versus without Lewy Bodies. Clin. Auton. Res. 2021, 31, 677–684; (c) McKeith, I. G.; Boeve, B. F.; Dickson, D. W.; Halliday, G.; Taylor, J. P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C. G.; et al. Diagnosis and Management of Dementia with Lewy Bodies. Neurology 2017, 89, 88–100; (d) Krismer, F.; Wenning, G. K. Multiple System Atrophy: Insights into a Rare and Debilitating Movement Disorder. Nat. Rev. Neurol. 2017, 13, 232–243; (e) Ayers, J. I.; Lee, J.; Monteiro, O.; Woerman, A. L.; Lazar, A. A.; Condello, C.; Paras, N. A.; Prusiner, S. B. Different α-Synuclein Prion Strains Cause Dementia with Lewy Bodies and Multiple System Atrophy. Proc. Natl. Acad. Sci. U. S. A. 2022, 119, e2113489119.
- 2(a) Wilson, D. M.; Cookson, M. R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D. M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714; (b) Jellinger, K. A. Neuropathological Spectrum of Synucleinopathies. Mov. Disord. 2003, 18, 2–12.
- 3(a) Burré, J.; Sharma, M.; Südhof, T. C. Cell Biology and Pathophysiology of α-Synuclein. Cold Spring Harbor Perspect. Med. 2018, 8, a024091; (b) Eliezer, D.; Kutluay, E.; Bussell, R.; Browne, G. Conformational Properties of α-Synuclein in Its Free and Lipid-associated States J. Mol. Biol. 2001, 307, 1061–1073; (c) Weinreb, P. H.; Zhen, W.; Poon, A. W.; Conway, K. A.; Lansbury, P. T. NACP, a Protein Implicated in Alzheimer's Disease and Learning, Is Natively Unfolded. Biochemistry 1996, 35, 13709–13715.
- 4Fusco, G.; De Simone, A.; Gopinath, T.; Vostrikov, V.; Vendruscolo, M.; Dobson, C. M.; Veglia, G. Direct Observation of the Three Regions in α-Synuclein that Determine Its Membrane-bound Behaviour. Nat. Commun. 2014, 5, 3827.
- 5(a) Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M. R.; Südhof, T. C. α-Synuclein Promotes SNARE-Complex Assembly in vivo and in vitro. Science 2010, 329, 1663–1667; (b) Lautenschläger, J.; Stephens, A. D.; Fusco, G.; Ströhl, F.; Curry, N.; Zacharopoulou, M.; Michel, C. H.; Laine, R.; Nespovitaya, N.; Fantham, M.; et al. C-terminal Calcium Binding of α-Synuclein Modulates Synaptic Vesicle Interaction. Nat. Commun. 2018, 9, 712; (c) Gitler, A. D.; Bevis, B. J.; Shorter, J.; Strathearn, K. E.; Hamamichi, S.; Su, L. J.; Caldwell, K. A.; Caldwell, G. A.; Rochet, J. C.; McCaffery, J. M.; et al. The Parkinson's Disease Protein α-Synuclein Disrupts Cellular Rab Homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 145–150; (d) Cooper, A. A.; Gitler, A. D.; Cashikar, A.; Haynes, C. M.; Hill, K. J.; Bhullar, B.; Liu, K.; Xu, K.; Strathearn, K. E.; Liu, F.; et al. α-Synuclein Blocks ER-Golgi Traffic and Rab1 Rescues Neuron Loss in Parkinson's Models. Science 2006, 313, 324–328; (e) Ullman, O.; Fisher, C. K.; Stultz, C. M. Explaining the Structural Plasticity of α-Synuclein. J. Am. Chem. Soc. 2011, 133, 19536–19546; (f) Uversky, V. N.; Li, J.; Souillac, P.; Millett, I. S.; Doniach, S.; Jakes, R.; Goedert, M.; Fink, A. L. Biophysical Properties of the Synucleins and Their Propensities to Fibrillate: Inhibition of α-Synuclein assembly by β- and γ-Synucleins. J. Biol. Chem. 2002, 277, 11970–11978; (g) Bartels, T.; Choi, J. G.; Selkoe, D. J. α-Synuclein Occurs Physiologically as a Helically Folded Tetramer that Resists Aggregation. Nature 2011, 477, 107–110; (h) Maltsev, A. S.; Chen, J.; Levine, R. L.; Bax, A. Site-Specific Interaction between α-Synuclein and Membranes Probed by NMR-Observed Methionine Oxidation Rates. J. Am. Chem. Soc. 2013, 135, 2943–2946.
- 6(a) Reynolds, N. P.; Soragni, A.; Rabe, M.; Verdes, D.; Liverani, E.; Handschin, S.; Riek, R.; Seeger, S. Mechanism of Membrane Interaction and Disruption by α-Synuclein. J. Am. Chem. Soc. 2011, 133, 19366–19375; (b) Van Maarschalkerweerd, A.; Vetri, V.; Langkilde, A. E.; Foderà, V.; Vestergaard, B. Protein/Lipid Coaggregates are Formed During α-Synuclein-induced Disruption of Lipid Bilayers. Biomacromolecules 2014, 15, 3643–3654; (c) Shahmoradian, S. H.; Lewis, A. J.; Genoud, C.; Hench, J.; Moors, T. E.; Navarro, P. P.; Castaño Díez, D.; Schweighauser, G.; Graff Meyer, A.; Goldie, K. N.; et al. Lewy Pathology in Parkinson's Disease Consists of Crowded Organelles and Lipid Membranes. Nat. Neurosci. 2019, 22, 1099–1109; (d) Lee, H. J.; Choi, C.; Lee, S. J. Membrane-bound α-Synuclein Has a High Aggregation Propensity and the Ability to Seed the Aggregation of the Cytosolic Form. J. Biol. Chem. 2002, 277, 671–678; (e) Fusco, G.; Chen, S. W.; Williamson, P. T. F.; Cascella, R.; Perni, M.; Jarvis, J. A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural Basis of Membrane Disruption and Cellular Toxicity by α-Synuclein Oligomers. Science 2017, 358, 1440–1443; (f) Hoover, B. M.; Shen, Z.; Gahan, C. G.; Lynn, D. M.; Van Lehn, R. C.; Murphy, R. M. Membrane Remodeling and Stimulation of Aggregation Following α-Synuclein Adsorption to Phosphotidylserine Vesicles. J. Phys. Chem. B 2021, 125, 1582–1594.
- 7Vergniory, N. B.; Roberts, R. F.; Martins, R. W.; Abarrategui, J. A. Alpha-synuclein Oligomers: a New Hope. Acta Neuropathol. 2017, 134, 819–838.
- 8(a) Cole, N. B.; Murphy, D. D.; Grider, T.; Rueter, S.; Brasaemle, D.; Nussbaum, R. L. Lipid Droplet Binding and Oligomerization Properties of the Parkinson's Disease Protein α-Synuclein. J. Biol. Chem. 2002, 277, 6344–6352; (b) Bell, R.; Vendruscolo, M. Modulation of the Interactions Between α-Synuclein and Lipid Membranes by Post- translational Modifications. Front. Neurol. 2021, 12, 661117; (c) Galvagnion, C.; Buell, A. K.; Meisl, G.; Michaels, T. C. T.; Vendruscolo, M.; Knowles, T. P. J.; Dobson, C. M. Lipid Vesicles Trigger α-Synuclein Aggregation by Stimulating Primary Nucleation. Nat. Chem. Biol. 2015, 11, 229–234; (d) Dou, T.; Kurouski, D. Phosphatidylcholine and Phosphatidylserine Uniquely Modify the Secondary Structure of α-Synuclein Oligomers Formed in Their Presence at the Early Stages of Protein Aggregation. ACS Chem. Neurosci. 2022, 13, 2380–2385.
- 9Iyer, A.; Claessens, M. M. A. E. Disruptive Membrane Interactions of Alpha-synuclein Aggregates. Biochim. Biophys. Acta, Proteins Proteomics 2019, 1867, 468–482.
- 10(a) Zhu, M.; Fink, A. L. Lipid Binding Inhibits α-Synuclein Fibril Formation. J. Mol. Biol. 2003, 278, 16873–16877; (b) Galvagnion, C.; Brown, J. W. P.; Ouberai, M. M.; Flagmeier, P.; Vendruscolo, M.; Buell, A. K.; Sparr, E.; Dobson, C. M. Chemical Properties of Lipids Strongly Affect the Kinetics of the Membrane-induced Aggregation of α-Synuclein. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 7065–7070; (c) Zhu, M.; Fink, A. L. Lipid Binding Inhibits α-Synuclein Fibril Formation. J. Biol. Chem. 2003, 278, 16873–16877.
- 11(a) Yang, J. A.; Lin, W.; Woods, W. S.; George, J. M.; Murphy, C. J. α-Synuclein's Adsorption, Conformation, and Orientation on Cationic Gold Nanoparticle Surfaces Seeds Global Conformation Change. J. Phys. Chem. B 2014, 118, 3559–3571; (b) Mohammad Beigi, H.; Shojaosadati, S. A.; Marvian, A. T.; Pedersen, J. N.; Klausen, L. H.; Christiansen, G.; Pedersen, J. S.; Dong, M.; Morshedi, D.; Otzen, D. E. Strong Interactions with Polyethylenimine-coated Human Serum Albumin Nanoparticles (PEI-HSA NPs) Alter α-Synuclein Conformation and Aggregation Kinetics. Nanoscale 2015, 7, 19627–19640.
- 12Dou, T.; Zhou, L.; Kurouski, D. Unravelling the Structural Organization of Individual α-Synuclein Oligomers Grown in the Presence of Phospholipids. J. Phys. Chem. Lett. 2021, 12, 4407–4414.
- 13(a) Martial, B.; Lefèvre, T.; Buffeteau, T.; Auger, M. Vibrational Circular Dichroism Reveals Supramolecular Chirality Inversion of α-Synuclein Peptide Assemblies upon Interactions with Anionic Membranes. ACS Nano 2019, 13, 3232–3242; (b) Dasari, A. K. R.; Dillard, L.; Yi, S.; Viverette, E.; Hojjatian, A.; Sengupta, U.; Kayed, R.; Taylor, K. A.; Borgnia, M. J.; Lim, K. H. Untwisted α-Synuclein Filaments Formed in the Presence of Lipid Vesicles. Biochemistry 2022, 61, 1766–1773.
- 14(a) Maeda, Y.; Yamamoto, H.; Kitano, H. Self-Assembled Monolayers as Novel Biomembrane Mimetics. 1. Characterization of Cytochrome c Bound to Self-Assembled Monolayers on Silver by Surface-Enhanced Resonance Raman Spectroscopy. J. Phys. Chem. 1995, 99, 4837–4841; (b) Lopez, G. P.; Albers, M. W.; Schreiber, S. L.; Carroll, R.; Peralta, E.; Whitesides, G. M. Convenient Methods for Patterning the Adhesion of Mammalian Cells to Surfaces Using Self-assembled Monolayers of Alkanethiolates on Gold. J. Am. Chem. Soc. 1993, 115, 5877–5878; (c) Spinke, J.; Liley, M.; Guder, H. J.; Angermaier, L.; Knoll, W. Molecular Recognition at Self-assembled Monolayers: the Construction of Multicomponent Multilayers. Langmuir 1993, 9, 1821–1825; (d) Prime, K. L.; Whitesides, G. M. Adsorption of Proteins onto Surfaces Containing End-attached Oligo(ethylene oxide): a Model System Using Self-assembled Monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721.
- 15Álvarez, Y. D.; Fauerbach, J. A.; Pellegrotti, J. V.; Jovin, T. M.; Jares Erijman, E. A.; Stefani, F. D. Influence of Gold Nanoparticles on the Kinetics of α-Synuclein Aggregation. Nano Lett. 2013, 13, 6156–6163.
- 16(a) Burley, S. K.; Petsko, G. A. Aromatic-aromatic Interaction: a Mechanism of Protein Structure Stabilization. Science 1985, 229, 23–28; (b) Moreira, I. S.; Martins, J. M.; Ramos, R. M.; Fernandes, P. A.; Ramos, M. J. Understanding the Importance of the Aromatic Amino-acid Residues as Hot-spots. Biochim. Biophys. Acta, Proteins Proteomics 2013, 1834, 404–414.
- 17(a) Rosendahl, S. M.; Burgess, I. J. Electrochemical and Infrared Spectroscopy Studies of 4-Mercaptobenzoic Acid SAMs on Gold Surfaces. Electrochim. Acta 2008, 53, 6759–6767; (b) Creager, S. E.; Steiger, C. M. Conformational Rigidity in a Self-Assembled Monolayer of 4-Mercaptobenzoic Acid on Gold. Langmuir 1995, 11, 1852–1854; (c) Wilson, E. B. The Normal Modes and Frequencies of Vibration of the Regular Plane Hexagon Model of the Benzene Molecule. Phys. Rev. 1934, 45, 706–714; (d) Osawa, M.; Matsuda, N.; Yoshii, K.; Uchida, I. Charge Transfer Resonance Raman Process in Surface- enhanced Raman Scattering from p-Aminothiophenol Adsorbed on Silver: Herzberg-Teller Contribution. J. Phys. Chem. 1994, 98, 12702–12707.
- 18Osawa, M.; Ataka, K.; Yoshii, K.; Nishikawa, Y. Surface-Enhanced Infrared Spectroscopy: The Origin of the Absorption Enhancement and Band Selection Rule in the Infrared Spectra of Molecules Adsorbed on Fine Metal Particles. Appl. Spectrosc. 1993, 47, 1497–1502.
- 19Li, J.; Uversky, V. N.; Fink, A. L. Effect of Familial Parkinson's Disease Point Mutations A30P and A53T on the Structural Properties, Aggregation, and Fibrillation of Human α-Synuclein. Biochemistry 2001, 40, 11604–11613.
- 20(a) Yu, Y.; Handa, S.; Yajima, T.; Futamata, M. Flocculation of Ag Nanoparticles Elucidating Adsorbed p-Mercaptobenzoic Acid by Surface Enhanced Raman Scattering. Chem. Phys. Lett. 2013, 560, 49–54; (b) Gu, Z.; Tian, S.; Zhou, Q.; Wei, W.; Zhao, L.; Li, X.; Zheng, J. Surface Enhanced Raman Scattering of Molecules Related to Highly Ordered Gold Cavities. J. Raman Spectrosc. 2013, 44, 1682–1688; (c) Bishnoi, S. W.; Rozell, C. J.; Levin, C. S.; Gheith, M. K.; Johnson, B. R.; Johnson, D. H.; Halas, N. J. All-Optical Nanoscale pH Meter. Nano Lett. 2006, 6, 1687–1692; (d) Handa, S.; Yu, Y.; Futamata, M. Adsorbed State of p-Mercaptobenzoic Acid on Silver Nanoparticles. Vib. Spectrosc. 2014, 72, 128–133.
- 21Wu, K. P.; Weinstock, D. S.; Narayanan, C.; Levy, R. M.; Baum, J. Structural Reorganization of α-Synuclein at Low pH Observed by NMR and REMD Simulations. J. Mol. Biol. 2009, 391, 784–796.
- 22Tang, C. Y.; Allen, H. C. Ionic Binding of Na+ versus K+ to the Carboxylic Acid Headgroup of Palmitic Acid Monolayers Studied by Vibrational Sum Frequency Generation Spectroscopy. J. Phys. Chem. A 2009, 113, 7383–7393.
- 23Sharp, J. S.; Forrest, J. A.; Jones, R. A. L. Surface Denaturation and Amyloid Fibril Formation of Insulin at Model Lipid−Water Interfaces. Biochemistry 2002, 41, 15810–15819.
- 24(a) Rabe, M.; Verdes, D.; Seeger, S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106; (b) Brightbill, E. L.; Young, K. T.; Gezahagne, H. F.; Jin, D. S.; Hitchcock, B.; Vogel, E. M. Protein Interactions with Chemical Vapor Deposited Graphene Modified by Substrate. 2D Mater. 2021, 8, 025015.
- 25Forbrig, E.; Staffa, J. K.; Salewski, J.; Mroginski, M. A.; Hildebrandt, P.; Kozuch, J. Monitoring the Orientational Changes of Alamethicin during Incorporation into Bilayer Lipid Membranes. Langmuir 2018, 34, 2373–2385.
- 26Vitali, M.; Rigamonti, V.; Natalello, A.; Colzani, B.; Avvakumova, S.; Brocca, S.; Santambrogio, C.; Narkiewicz, J.; Legname, G.; Colombo, M.; et al. Conformational Properties of Intrinsically Disordered Proteins Bound to the Surface of Silica Nanoparticles. Biochim. Biophys. Acta, Gen. Subj. 2018, 1862, 1556–1564.
- 27(a) Ramer, G.; Ruggeri, F. S.; Levin, A.; Knowles, T. P. J.; Centrone, A. Determination of Polypeptide Conformation with Nanoscale Resolution in Water. ACS Nano 2018, 12, 6612–6619; (b) Yoneda, J. S.; Miles, A. J.; Araujo, A. P. U.; Wallace, B. A. Differential Dehydration Effects on Globular Proteins and Intrinsically Disordered Proteins during Film Formation. Protein Sci. 2017, 26, 718–726.
- 28(a) Jackson, M.; Mantsch, H. H. The Use and Misuse of FTIR Spectroscopy in the Determination of Protein Structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120; (b) Seo, J.; Hoffmann, W.; Warnke, S.; Huang, X.; Gewinner, S.; Schöllkopf, W.; Bowers, M. T.; von Helden, G.; Pagel, K. An Infrared Spectroscopy Approach to Follow β-Sheet Formation in Peptide Amyloid Assemblies. Nat. Chem. 2017, 9, 39–44; (c) Zhou, L.; Kurouski, D. Structural Characterization of Individual α-Synuclein Oligomers Formed at Different Stages of Protein Aggregation by Atomic Force Microscopy-Infrared Spectroscopy. Anal. Chem. 2020, 92, 6806–6810; (d) Uversky, V. N.; Li, J.; Fink, A. L. Evidence for a Partially Folded Intermediate in α-Synuclein Fibril Formation. J. Biol. Chem. 2001, 276, 10737–10744; (e) Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta, Bioenerg. 2007, 1767, 1073–1101.
- 29Celej, M. S.; Sarroukh, R.; Goormaghtigh, E.; Fidelio, G. D.; Ruysschaert, J. M.; Raussens, V. Toxic Prefibrillar α-Synuclein Amyloid Oligomers Adopt a Distinctive Antiparallel β-Sheet Structure. Biochem. J. 2012, 443, 719–726.
- 30(a) Croke, R. L.; Patil, S. M.; Quevreaux, J.; Kendall, D. A.; Alexandrescu, A. T. NMR Determination of pKa Values in α-Synuclein. Protein Sci. 2011, 20, 256–269; (b) Dedmon, M. M.; Larsen, K. L.; Christodoulou, J.; Vendruscolo, M.; Dobson, C. M. Mapping Long- range Interactions in α-Synuclein using Spin-Label NMR and Ensemble Molecular Dynamics Simulations. J. Am. Chem. Soc. 2005, 127, 476–477; (c) Bertoncini, C. W.; Jung, Y. S.; Fernandez, C. O.; Hoyer, W.; Griesinger, C.; Jovin, T. M.; Zweckstetter, M. Release of Long- range Tertiary Interactions Potentiates Aggregation of Natively Unstructured α-Synuclein. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 1430–1435.
- 31(a) George, J. M.; Jin, H.; Woods, W. S.; Clayton, D. F. Characterization of a Novel Protein Regulated During the Critical Period for Song Learning in the Zebra Finch. Neuron 1995, 15, 361–372; (b) Davidson, W. S.; Jonas, A.; Clayton, D. F.; George, J. M. Stabilization of α-Synuclein Secondary Structure upon Binding to Synthetic Membranes. J. Biol. Chem. 1998, 273, 9443–9449.
- 32Yang, J. A.; Johnson, B. J.; Wu, S.; Woods, W. S.; George, J. M.; Murphy, C. J. Study of Wild-Type α-Synuclein Binding and Orientation on Gold Nanoparticles. Langmuir 2013, 29, 4603–4615.
- 33(a) Bisaglia, M.; Tessari, I.; Pinato, L.; Bellanda, M.; Giraudo, S.; Fasano, M.; Bergantino, E.; Bubacco, L.; Mammi, S. A Topological Model of the Interaction between α-Synuclein and Sodium Dodecyl Sulfate Micelles. Biochemistry 2005, 44, 329–339; (b) Jao, C. C.; Der Sarkissian, A.; Chen, J.; Langen, R. Structure of Membrane-bound α-Synuclein Studied by Site-directed Spin Labeling. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 8331–8336; (c) Bussell, R.; Eliezer, D. A Structural and Functional Role for 11-mer Repeats in α-Synuclein and Other Exchangeable Lipid Binding Proteins. J. Mol. Biol. 2003, 329, 763–778.
- 34Camino, J. D.; Gracia, P.; Chen, S. W.; Sot, J.; de la Arada, I.; Sebastián, V.; Arrondo, J. L. R.; Goñi, F. M.; Dobson, C. M.; Cremades, N. The Extent of Protein Hydration Dictates the Preference for Heterogeneous or Homogeneous Nucleation Generating either Parallel or Antiparallel β-Sheet α-Synuclein Aggregates. Chem. Sci. 2020, 11, 11902–11914.
- 35(a) Theillet, F. X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H. M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural Disorder of Monomeric α-Synuclein Persists in Mammalian Cells. Nature 2016, 530, 45–50; (b) Farzadfard, A.; Pedersen, J. N.; Meisl, G.; Somavarapu, A. K.; Alam, P.; Goksøyr, L.; Nielsen, M. A.; Sander, A. F.; Knowles, T. P. J.; Pedersen, J. S.; et al. The C-terminal Tail of α-Synuclein Protects against Aggregate Replication but is Critical for Oligomerization. Commun. Biol. 2022, 5, 123.
- 36Giasson, B. I.; Murray, I. V. J.; Trojanowski, J. Q.; Lee, V. M. Y. A Hydrophobic Stretch of 12 Amino Acid Residues in the Middle of α-Synuclein Is Essential for Filament Assembly. J. Mol. Biol. 2001, 276, 2380–2386.
- 37(a) Pálmadóttir, T.; Malmendal, A.; Leiding, T.; Lund, M.; Linse, S. Charge Regulation during Amyloid Formation of α-Synuclein. J. Am. Chem. Soc. 2021, 143, 7777–7791; (b) Schweighauser, M.; Shi, Y.; Tarutani, A.; Kametani, F.; Murzin, A. G.; Ghetti, B.; Matsubara, T.; Tomita, T.; Ando, T.; Hasegawa, K.; et al. Structures of α-Synuclein Filaments from Multiple System Atrophy. Nature 2020, 585, 464–469; (c) Miake, H.; Mizusawa, H.; Iwatsubo, T.; Hasegawa, M. Biochemical Characterization of the Core Structure of α-Synuclein Filaments. J. Mol. Biol. 2002, 277, 19213–19219.
- 38Guerrero Ferreira, R.; Taylor, N. M. I.; Arteni, A. A.; Kumari, P.; Mona, D.; Ringler, P.; Britschgi, M.; Lauer, M. E.; Makky, A.; Verasdonck, J.; et al. Two New Polymorphic Structures of Human Full-length Alpha- synuclein Fibrils Solved by Cryo-electron Microscopy. eLife 2019, 8, e48907.
- 39Tuttle, M. D.; Comellas, G.; Nieuwkoop, A. J.; Covell, D. J.; Berthold, D. A.; Kloepper, K. D.; Courtney, J. M.; Kim, J. K.; Barclay, A. M.; Kendall, A.; et al. Solid-state NMR Sstructure of a Pathogenic Fibril of Full-length Human α-Synuclein. Nat. Struct. Mol. Biol. 2016, 23, 409–415.
- 40Brown, G. C.; Neher, J. J. Microglial Phagocytosis of Live Neurons. Nat. Rev. Neurosci. 2014, 15, 209–216.
- 41(a) Bornhorst, J. A.; Falke, J. J. Purification of Proteins Using Polyhistidine Affinity Tags. In Methods in Enzymology, Vol. 326, Academic Press, Amsterdam, 2000, pp. 245–254; (b) Meuvis, J.; Gerard, M.; Desender, L.; Baekelandt, V.; Engelborghs, Y. The Conformation and the Aggregation Kinetics of α-Synuclein Depend on the Proline Residues in Its C-Terminal Region. Biochemistry 2010, 49, 9345–9352; (c) Gräslund, S.; Nordlund, P.; Weigelt, J.; Hallberg, B. M.; Bray, J.; Gileadi, O.; Knapp, S.; Oppermann, U.; Arrowsmith, C.; Hui, R.; et al. Protein Production and Purification. Nat. Methods 2008, 5, 135–146.
- 42Li, S.; Wu, L.; Zhang, X.; Jiang, X. The Structure of Water Bonded to Phosphate Groups at the Electrified Zwitterionic Phospholipid Membranes/Aqueous Interface. Angew. Chem. Int. Ed. 2020, 59, 6627–6630.
- 43(a) Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining Information about Protein secondary Structures in Aqueous Solution Using Fourier Transform IR Spectroscopy. Nat. Protoc. 2015, 10, 382–396;
(b) Natalello, A.; Ami, D.; Doglia, S. M. Fourier Transform Infrared Spectroscopy of Intrinsically Disordered Proteins. In Intrinsically Disordered Protein Analysis, Vol. 1, Humana Press, Totowa, 2012, pp. 229–244.
10.1007/978-1-61779-927-3_16 Google Scholar




