Alkyl Radical Initiated Cyclization/Cascade for Synthesizing Lactam-Substituted Alkyl Sulfones
Corresponding Author
Li-Jun Wu
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorKai-Yi Zhang
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorPei Yang
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorCorresponding Author
Chuan-Chong Peng
School of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorJin-Hui Liu
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorQing Li
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorDa-Zhi Sun
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorCorresponding Author
Shuangfeng Yin
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorCorresponding Author
Li-Jun Wu
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorKai-Yi Zhang
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorPei Yang
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorCorresponding Author
Chuan-Chong Peng
School of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorJin-Hui Liu
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorQing Li
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorDa-Zhi Sun
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
Search for more papers by this authorCorresponding Author
Shuangfeng Yin
College of Sciences, Central South University of Forestry and Technology, Changsha, Hunan, 410004 China
E-mail: [email protected], [email protected], [email protected]Search for more papers by this authorComprehensive Summary
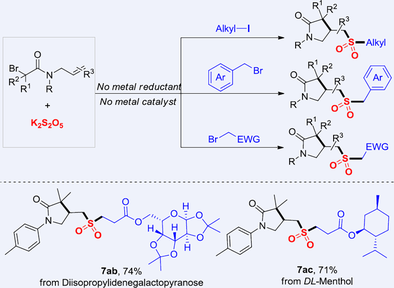
An alkyl radical initiated cyclization/tandem reaction of alkyl bromides and alkyl electrophiles by using potassium metabisulphite (K2S2O5) as a connector is developed for the synthesis of various lactam-substituted alkyl sulfones. Notably, this process does not require a metal catalyst or metal powder reductant, highlighting its environmentally friendly features. The reaction demonstrates outstanding substrate adaptability and a high tolerance towards diverse functional groups. Furthermore, the biologically active molecules and commercially available drugs with a late-stage modification are also highly compatible with this transformation. Mechanistic studies revealed that the reaction proceeds through a single-step process involving intramolecular radical cyclization, "SO2" insertion, and external alkyl incorporation.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400154-sup-0001-Supinfo.pdfPDF document, 7.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Das, P.; Delost, M. D.; Qureshi, M. H.; Smith, D. T.; Nijardarson, J. T. A Survey of the Structures of US FDA Approved Combination Drugs. J. Med. Chem. 2019, 62, 4265–4311; (b) Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. Data-Mining for Sulfur and Fluorine: An Evaluation of Pharmaceuticals to Reveal Opportunities for Drug Design and Discovery. J. Med. Chem. 2014, 57, 2832–2842; (c) Scott, K. A.; Njardarson, J. T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5.
- 2(a) Bohl, C. E.; Gao, W.; Miller, D. D.; Bell, C. E.; Dalton, J. T. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 6201–6206; (b) Liu, K. K.-C.; Bailey, S.; Dinh, D. M.; Lam, H.; Li, C.; Well, P. A.; Yin, M.-J.; Zou, A. Conformationally-restricted cyclic sulfones as potent and selective mTOR kinase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 5114–5117; (c) Fischli, W.; Clozel, J.-P.; Breu, V.; Buchmann, S.; Mathews, S.; Stadler, H.; Vieira, E.; Wostl, W. Ciprokiren (Ro 44-9375). A renin inhibitor with increasing effects on chronic treatment. Hypertension 1994, 24, 163–169; (d) Nicholls, P. H.; Bromilow, R. H.; Addiscott, T. M. Measured and simulated behaviour of fluometuron, aldoxycarb and chloride ion in a fallow structured soil. Pestic. Sci. 1982, 13, 475–483.
- 3(a) Foote, K. M.; Blades, K.; Cronin, A.; Fillery, S.; Guichard, S. S.; Hassall, L.; Hichson, I.; Jacq, X.; Jewsbury, P. J.; McGuire, T. M.; Nissink, J. W. M.; Odedra, R.; Page, K.; Perkins, P.; Suleman, A.; Tam, K.; Thommes, P.; Broadhurst, R.; Wood, C. Discovery of 4-{4-[(3R)-3- Methylmorpholin-4-yl]-6-[1-(methylsulfonyl)cyclopropyl]pyrimidin-2- yl}-1H-indole (AZ20): A Potent and Selective Inhibitor of ATR Protein Kinase with Monotherapy in vivo Antitumor Activity. J. Med. Chem. 2013, 56, 2125–2138; (b) Riches, A.; Hart, C. J. S.; Trenholme, K. R.; Skinner-Adams, T. S. Anti-Giardia Drug Discovery: Current Status and Gut Feelings. J. Med. Chem. 2020, 63, 13330–13354; (c) Keating, G. M. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 2017, 77, 459–472.
- 4(a) Aziz, J.; Messaoudi, S.; Alami, M.; Hamze, A. Sulfinate derivatives: dual and versatile partners in organic synthesis. Org. Biomol. Chem. 2014, 12, 9743–9759; (b) Qiu, G.; Zhou, K.; Gao, L.; Wu, J. Insertion of sulfur dioxide via a radical process: an efficient route to sulfonyl compounds. Org. Chem. Front. 2018, 5, 691–705; (c) Ye, S.; Qiu, G.; Wu, J. Inorganic sulfites as the sulfur dioxide surrogates in sulfonylation reactions. Chem. Commun. 2019, 55, 1013–1019; (d) Zeng, D.; Wang, M.; Deng, W.-P.; Jiang, X. The same oxygenation-state introduction of hypervalent sulfur under transition-metal-free conditions. Org. Chem. Front. 2020, 7, 3956–3966; (e) He, J.; Chen, G.; Zhang, B.; Li, Y.; Chen, J.-R.; Zhang, B.; Li, Y.; Chen, J.-R.; Xiao, W.-J.; Liu, F.; Li, C. Catalytic Decarboxylative Radical Sulfonylation. Chem 2020, 6, 1149–1159; (f) Zhang, Q.; Dong, D.; Zi, W. Palladium-Catalyzed Regio- and Enantioselective Hydrosulfonylation of 1,3-Dienes with Sulfinic Acids: Scope, Mechanism, and Origin of Selectivity. J. Am. Chem. Soc. 2020, 142, 15860–15869; (g) Deng, R.; Wu, S.; Mou, C.; Liu, J.; Zheng, P.; Zhang, X.; Chi, Y. R. Carbene-Catalyzed Enantioselective Sulfonylation of Enone Aryl Aldehydes: A New Mode of Breslow Intermediate Oxidation. J. Am. Chem. Soc. 2022, 144, 5441–5449; (h) Xu, K.; Li, L.; Yan, W.; Wu, Y.; Wang, Z.; Zhang, S. Dual roles of sulfonyl hydrazides in the catalyst-free sulfonylation of unsaturated benzylic alcohols in water. Green Chem. 2017, 19, 4494–4497; (i) Liu, W.; Zhao, Y.; Ma, W.; Chai, Z.; Liang, Y.; Lv, Z.-J.; Xu, L.; Wei, J.; Zhang, W.-X. Cross-carbanion coupling at a rare-earth center. Cell Rep. Phys. Sci. 2023, 4, 101479; (j) Li, Y.; Liu, J.-B.; He, F.-S.; Wu, J. Photoredox-Catalyzed Functionalization of Alkenes with Thiourea Dioxide: Construction of Alkyl Sulfones or Sulfonamides. Chin. J. Chem. 2020, 38, 361–366; (k) Song, Q.; Zhao, H.; Sun, Y.; Jiang, H.; Zhang, M. Direct C(sp3)–H Sulfonylation of Xanthene Derivatives with Sodium Sulfinates by Oxidative Copper Catalysis. Chin. J. Chem. 2022, 40, 371–377; (l) Hu, L.-P.; Zhang, D.-R.; Huang, X.-H.; Liu, F.-L.; Li, X.; Teng, M.-Y.; Huang, G.-L. Metal-Free Arylsulfonyl Radical Triggered Cascade Cyclization of Phenyl-Linked 1,6-Enynes: Synthesis of 2,3-Dihydro-1H-indenes and 10a,11-Dihydro-10H-benzo[b]fluorines. Chin. J. Chem. 2022, 40, 2756–2762; (m) Song, T.; Zhang, Y.; Wang, C.; Li, Y.; Yang, Y. Photocatalytic Aerobic Oxysulfonylation of Alkynes to Access β-Keto Sulfones Catalyzed by OVs-N-Nb2O5. Chin. J. Chem. 2022, 40, 2618–2624.
- 5(a) Wang, Y.; Zhao, Y.; Cai, C.; Wang, L.; Gong, H. Dioxygen-Triggered Oxosulfonylation/Sulfonylation of Terminal Olefins toward β-Keto Sulfones/Sulfones. Org. Lett. 2021, 23, 8296–8301; (b) Zhu, H.; Shen, Y.; Deng, Q.; Chen, J.; Tu, T. Acenaphthoimidazolylidene Gold Complex-Catalyzed Alkylsulfonylation of Boronic Acids by Potassium Metabisulfite and Alkyl Halides: A Direct and Robust Protocol to Access Sulfones. ACS Catal. 2017, 7, 4655–4659; (c) Zhang, J.; Zhou, K.; Qiu, G.; Wu, J. Photoinduced synthesis of allylic sulfones using potassium metabisulfite as the source of sulfur dioxide. Org. Chem. Front. 2019, 6, 36–40; (d) Zhang, J.; An, Y.; Wu, J. Vicinal Difunctionalization of Alkenes through a Multicomponent Reaction with the Insertion of Sulfur Dioxide. Chem. Eur. J. 2017, 23, 9477–9480; (e) Xu, K.; Khakyzadeh, V.; Bury, T.; Breit, B. Direct Transformation of Terminal Alkynes to Branched Allylic Sulfones. J. Am. Chem. Soc. 2014, 136, 16124–16127; (f) Yan, Q.; Xiao, G.; Wang, Y.; Zi, G.; Zhang, Z.; Hou, G. Highly Efficient Enantioselective Synthesis of Chiral Sulfones by Rh-Catalyzed Asymmetric Hydrogenation. J. Am. Chem. Soc. 2019, 141, 1749–1756; (g) Hashim, F. M.; Kuo, Y.-H.; Stinger, B. L.; Palfey, B. A.; Martin, B. R. Tunable Heteroaromatic Sulfones Enhance in-Cell Cysteine Profiling. J. Am. Chem. Soc. 2020, 142, 1801–1810; (h) Song, H.; Zhang, X.; Chen, G.; He, X.; Lian, Z. Copper-Catalyzed 1,4-Trifluoromethylthio-Arylsulfonylation of 1,3-Enynes via the Insertion of Sulfur Dioxide. Org. Lett. 2023, 25, 5916–5921; (i) Shavnya, A.; Coffey, S. B.; Smith, A. C.; Mascitti, V. Palladium-Catalyzed Sulfination of Aryl and Heteroaryl Halides: Direct Access to Sulfones and Sulfonamides. Org. Lett. 2013, 15, 6226–6229; (j) Xiao, F.; Liu, C.; Wang, D.; Huang, H.; Deng, G.-J. Concise synthesis of ketoallyl sulfones through an iron-catalyzed sequential four-component assembly. Green Chem. 2018, 20, 973–977; (k) Zhao, F.; Tan, Q.; Wang, D.; Deng, G.-J. Metal- and solvent-free direct C–H thiolation of aromatic compounds with sulfonyl chlorides. Green Chem. 2020, 22, 427–432; (l) Liu, Z.-L.; Ye, Z.-P.; Liao, Z.; Lu, W.-D.; Guan, J.-P.; Gao, Z.-Y.; Chen, K.; Chen, X.-Q.; Xiang, H.-Y.; Yang, H. Photoinduced, Palladium-Catalyzed Enantioselective 1,2-Alkylsulfonylation of 1,3-Dienes. ACS Catal. 2024, 14, 3725–3732.
- 6(a) Maayan, G.; Popovitz-Biro, R.; Neumann, R. Micelle Directed Synthesis of Polyoxometalate Nanoparticles and Their Improved Catalytic Activity for the Aerobic Oxidation of Sulfides. J. Am. Chem. Soc. 2006, 128, 4968–4969; (b) Trost, B. M.; Braslau, R. Tetra-n-butylammonium oxone. Oxidations under anhydrous conditions. J. Org. Chem. 1988, 53, 532–537.
- 7(a) Ju, Y.; Kumar, D.; Varma, R. S. Revisiting Nucleophilic Substitution Reactions: Microwave-Assisted Synthesis of Azides, Thiocyanates, and Sulfones in an Aqueous Medium. J. Org. Chem. 2006, 71, 6697–6700; (b) Vennstra, G.; Zwaneburg, B. An Improved Synthesis of Sulfones using Tetrabutyl-ammonium Sulfinates. Synthesis 1975, 519–520.
- 8(a) Gulbe, K.; Turks, M. Synthesis of Sulfones via Ru(II)-Catalyzed Sulfination of Boronic Acids. J. Org. Chem. 2020, 85, 5660–5669; (b) Wolff, N.; Char, J.; Frogneux, X.; Cantat, T. Synthesis of Aromatic Sulfones from SO2 and Organosilanes Under Metal-free Conditions. Angew. Chem. Int. Ed. 2017, 56, 5616–5619; (c) Chen, J.-Q.; Liu, N.; Hu, Q.; Liu, J.; Wu, J.; Cai, Q.; Wu, J. Photocatalytic three-component radical cascade: a general route to heterocyclic-substituted alkyl sulfones. Org. Chem. Front. 2021, 8, 5316–5321; (d) Chen, M.; Sun, W.; Yang, J.; Yuan, L. L.; Chen, J.-Q.; Wu, J. Metal-free photosensitized aminosulfonylation of alkenes: a practical approach to β-amido sulfones. Green Chem. 2023, 25, 3857–3863.
- 9(a) Deeming, A. S.; Russell, C. J.; Hennessy, A. J.; Willis, M. C. DABSO- Based, Three-Component, One-Pot Sulfone Synthesism. Org. Lett. 2014, 16, 150–153; (b) Zheng, D.; Mao, R.; Li, Z.; Wu, J. A copper(I)- catalyzed three-component reaction of triethoxysilanes, sulfur dioxide, and alkyl halides. Org. Chem. Front. 2016, 3, 359–363; (c) Huang, J.; Liu, F.; Zeng, L.-H.; Li, S.; Chen, Z.; Wu, J. Accessing chiral sulfones bearing quaternary carbon stereocenters via photoinduced radical sulfur dioxide insertion and Truce–Smiles rearrangement. Nat. Commun. 2022, 13, 7081–7089; (d) Zhang, J.; Wang, X.; Wang, P.; Fang, J.; Li, S.; Wu, J. SO2-Insertion induced enantioselective oxysulfonylation to access β-chiral sulfones with quaternary carbon stereocenters. Sci. China Chem. 2024, 67, 908–913; (e) Chen, S.-Y.; Wang, Y.-S.; Han, X.; Zhang, Z.-D.; Li, Z.-K.; Lu, D.-L.; Li, S. Catalyst-free photoinduced radical sulfonylation/cyclization of unactivated alkenes toward sulfone-containing quinazolinones. Org. Chem. Front. 2024, 11, 1169–1174.
- 10(a) Li, Y. P.; Chen, S.; Wang, M.; Jiang, X. Sodium Dithionite-Mediated Decarboxylative Sulfonylation: Facile Access to Tertiary Sulfones. Angew. Chem. Int. Ed. 2020, 59, 8907–8911; (b) Chen, S.; Li, Y.; Wang, M.; Jiang, X. General sulfone construction via sulfur dioxide surrogate control. Green Chem. 2020, 22, 322–326; (c) Meng, Y.; Wang, M.; Jiang, X. F. Transition-Metal-Free Reductive Cross-Coupling Employing Metabisulfite as a Connector: General Construction of Alkyl-Alkyl Sulfones. CCS Chem. 2021, 3, 17–24.
- 11(a) Chung, C.-P.; Hsu, C.-Y.; Lin, J.-H.; Kuo, Y.-H.; Chiang, W.; Lin, Y.-L. Antiproliferative Lactams and Spiroenone from Adlay Bran in Human Breast Cancer Cell Lines. J. Agric. Food Chem. 2011, 59, 1185–1194; (b) Talele, T. T. Natural-Products-Inspired Use of the gem-Dimethyl Group in Medicinal Chemistry. J. Med. Chem. 2018, 61, 2166–2210; (c) Xie, Z.; Meng, Z.; Yang, X.; Duan, Y.; Wang, Q.; Liao, C. Factor XIa Inhibitors in Anticoagulation Therapy: Recent Advances and Perspectives. J. Med. Chem. 2023, 66, 5332–5363.
- 12(a) Meng, G.; Zhang, J.; Szostak, M. Acyclic Twisted Amides. Chem. Rev. 2021, 121, 12746–12783; (b) Wu, C.; Zhang, H.; Yu, B.; Chen, Y.; Ke, Z.; Guo, S.; Liu, Z. Lactate-Based Ionic Liquid Catalyzed Reductive Amination/Cyclization of Keto Acids under Mild Conditions: A Metal- Free Route To Synthesize Lactams. ACS Catal. 2017, 7, 7772–7776; (c) Rizzo, S.; Waldmann, H. Development of a Natural-Product-Derived Chemical Toolbox for Modulation of Protein Function. Chem. Rev. 2014, 114, 4621–4639; (d) Szostak, M.; Aube, J. Chemistry of Bridged Lactams and Related Heterocycles. Chem. Rev. 2013, 113, 5701–5765; (e) Nishiura, Y.; Gonzalez, K. J.; Cusumano, A. Q.; Stoltz, B. M. Enantioselective 1,3-Dipolar Cycloadditions of α-Methylene Lactams to Construct Spirocycles. Org. Lett. 2023, 25, 6469–6473.
- 13(a) Clark, A.; Wilson, P. Copper mediated atom transfer radical cyclisations with AIBN. Tetrahedron Lett. 2008, 49, 4848–4850; (b) Tsuchiya, N.; Nakashima, Y.; Hirata, G.; Nishikata, T. Atom-transfer radical cyclization of α-bromocarboxamides under organophotocatalytic conditions. Tetrahedron Lett. 2021, 69, 152952; (c) Schumacher, C.; Hernandez, J. G.; Bolm, C. Electro-Mechanochemical Atom Transfer Radical Cyclizations using Piezoelectric BaTiO3. Angew. Chem. Int. Ed. 2020, 59, 16357–16360; (d) Clark, A. J.; Geden, J. V.; Thom, S.; Wilson, P. Regiochemistry of Copper(I)-Mediated Cyclization Reactions of Halo-dienamides. J. Org. Chem. 2007, 72, 5923–5926; (e) Clark, A. J.; Collis, A. E. C.; Fox, D. J.; Halliwell, L. L.; James, N.; Reilly, R. K. O’.; Parekh, H.; Ross, A.; Sellars, A. B.; Willcock, H.; Wilson, P. Atom-Transfer Cyclization with CuSO4/KBH4: A Formal “Activators Generated by Electron Transfer” Process Also Applicable to Atom- Transfer Polymerization. J. Org. Chem. 2012, 77, 6778–6788.
- 14(a) Ye, Z.-P.; Xia, P.-J.; Liu, F.; Hu, Y.-Z.; Song, D.; Xiao, J.-A.; Huang, P.; Xiang, H.-Y.; Chen, X.-C.; Yang, H. Visible-Light-Induced, Catalyst-Free Radical Cross-Coupling Cyclization of N-Allylbromodifluoroacetamides with Disulfides or Diselenides. J. Org. Chem. 2020, 85, 5670–5682; (b) Li, M.; Jia, W.-Y.; Sun, G.-Q.; Gao, F.; Zhao, G.-X.; Qiu, Y.-F.; Wang, X.-C.; Liang, Y.-M.; Qua, Z.-J. Directed Copper-Catalyzed Tandem Radical Cyclization Reaction of Alkyl Bromides and Unactivated Olefins. Org. Lett. 2022, 24, 2738–2743; (c) Gou, X.-Y.; Li, Y.; Luan, Y.-Y.; Shi, W.-Y.; Wang, C.-T.; An, Y.; Zhang, B.-S.; Liang, Y.-M. Ruthenium-Catalyzed Radical Cyclization/meta-Selective C-H Alkylation of Arenes via σ-Activation Strategy. ACS Catal. 2021, 11, 4263–4270; (d) Zhang, Y.-C.; Chen, Y.; Sun, J.; Wang, J.-Y.; Zhou, M.-D. Visible- light-promoted Radical Cyclization/Arylation Cascade for the Construction of α,α-Difluoro-γ-Lactam-Fused Quinoxalin-2(1H)-Ones. Chin. J. Chem. 2022, 40, 713–718.
- 15(a) Peng, C.-C.; Long, F.; Zhang, K.-Y.; Hu, Y.-C.; Wu, L.-J. Copper(I)- Catalyzed Cross-Coupling of Arylsulfonyl Radicals with Diazo Compounds: Assembly of Arylsulfones. J. Org. Chem. 2022, 87, 12265–12273; (b) Zhang, K.-Y.; Long, F.; Peng, C.-C.; Liu, J.-H.; Hu, Y.-C.; Wu, L.-J. Multicomponent Sulfonylation of Alkenes to Access β-Substituted Arylsulfones. J. Org. Chem. 2023, 88, 3772–3780; (c) Zhang, K.-Y.; Long, F.; Peng, C.-C.; Liu, J.-H.; Wu, L.-J. Pd-Catalyzed Multicomponent Cross-Coupling of Allyl Esters with Alkyl Bromides and Potassium Metabisulfite: Access to Allylic Sulfones. Org. Lett. 2023, 25, 5817–5821.




