Redox-Active Dihydrophenazine-Based Macrocycle: Synthesis, Conformation-Adaptive Behavior and Host-Guest Complexation with Tetracyanoquinodimethane
Qiong-Yan Hong
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorBin Huang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorMeng-Xiang Wu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorLin Xu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorXiao-Li Zhao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorXueliang Shi
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Hai-Bo Yang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
E-mail: [email protected]Search for more papers by this authorQiong-Yan Hong
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorBin Huang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorMeng-Xiang Wu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorLin Xu
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorXiao-Li Zhao
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorXueliang Shi
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
Search for more papers by this authorCorresponding Author
Hai-Bo Yang
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, State Key Laboratory of Petroleum Molecular & Process Engineering, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, 200062 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
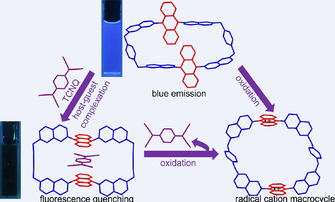
A novel macrocycle based on conformation-adaptive and electron-rich dihydrophenazine was designed and synthesized. On the one hand, the macrocycle showed host-guest interactions with tetracyanoquinodimethane (TCNQ) driving by charge transfer interaction between them. Meanwhile, host-guest complexation was accompanied by fluorescence quenching and conformational change of the macrocycle. On the other hand, the oxidation of the macrocycle resulted in its diradical cation analogue and induced the release of the guest molecule TCNQ, thereby accomplishing reversible binding dynamics. Therefore, this work well illustrates the chemical and structural versatility of dihydrophenazine in the synthesis of macrocycles and their host-guest chemistry.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400134-sup-0001-supinfo.pdfPDF document, 2.7 MB |
Appendix S1: Supporting information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Hiraoka, S.; Okamoto, T.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Okada, K. A Stable Radical-Substituted Radical Cation with Strongly Ferromagnetic Interaction: Nitronyl Nitroxide-Substituted 5,10-Diphenyl-5,10-dihydrophenazine Radical Cation. J. Am. Chem. Soc. 2004, 126, 58–59.
- 2 Dosso, J.; Prato, M. N,N-Diphenyl Dihydrophenazines: Using π-Extension to Access Dicationic Multifunctional Materials. Chem. Eur. J. 2023, 29, e202203637.
- 3 Song, W.; Ye, W.; Shi, L.; Huang, J.; Zhang, Z.; Mei, J.; Su, J.; Tian, H. Smart molecular butterfly: an ultra-sensitive and range-tunable ratiometric thermometer based on dihydrophenazines. Mater. Horiz. 2020, 7, 615–623.
- 4 Schorpp, M.; Heizmann, T.; Schmucker, M.; Rein, S.; Weber, S.; Krossing, I. Synthesis and Application of a Perfluorinated Ammoniumyl Radical Cation as a Very Strong Deelectronator. Angew. Chem. Int. Ed. 2020, 59, 9453–9459.
- 5 Jin, X.; Li, S.; Guo, L.; Hua, J.; Qu, D.-H.; Su, J.; Zhang, Z.; Tian, H. Interplay of Steric Effects and Aromaticity Reversals to Expand the Structural/Electronic Responses of Dihydrophenazines. J. Am. Chem. Soc. 2022, 144, 4883–4896.
- 6 Huang, B.; Kang, H.; Zhao, X.-L.; Yang, H.-B.; Shi, X. Redox Properties of N,N’-Disubstituted Dihydrophenazine and Dihydrodibenzo[a,c]- phenazine: The First Isolation of Their Crystalline Radical Cations and Dications. Cryst. Growth Des. 2022, 22, 3587–3593.
- 7 Dosso, J.; Bartolomei, B.; Demitri, N.; Cossío, F. P.; Prato, M. Phenanthrene-Extended Phenazine Dication: An Electrochromic Conformational Switch Presenting Dual Reactivity. J. Am. Chem. Soc. 2022, 144, 7295–7301.
- 8 Xie, G.; Bojanowski, N. M.; Brosius, V.; Wiesner, T.; Rominger, K.; Freudenberg, J.; Bunz, U. F. Stable N,N’-Diarylated Dihydrodiazaacene Radical Cations. Chem. Eur. J. 2021, 27, 1976–1980.
- 9 Theriot, J. C.; Lim, C. H.; Yang, H.; Ryan, M. D.; Musgrave, C. B.; Miyake, G. M. Organocatalyzed atom transfer radical polymerization driven by visible light. Science 2016, 352, 1082–1086.
- 10 Lim, C. H.; Ryan, M. D.; McCarthy, B. G.; Theriot, J. C.; Sartor, S. M.; Damrauer, N. H.; Musgrave, C. B.; Miyake, G. M. Intramolecular Charge Transfer and Ion Pairing in N,N’-Diaryl Dihydrophenazine Photoredox Catalysts for Efficient Organocatalyzed Atom Transfer Radical Polymerization. J. Am. Chem. Soc. 2017, 139, 348–355.
- 11 Li, L.; Su, Y.; Ji, Y.; Wang, P. A Long-Lived Water-Soluble Phenazine Radical Cation. J. Am. Chem. Soc. 2023, 145, 5778–5785.
- 12 Zhao, X.; Qiu, X.; Xue, H.; Liu, S.; Liang, D.; Yan, C.; Chen, W.; Wang, Y.; Zhou, G. Conjugated and Non-conjugated Polymers Containing Two- Electron Redox Dihydrophenazines for Lithium-Organic Batteries. Angew. Chem. Int. Ed. 2023, 62, e202216713.
- 13 Masuda, Y.; Kuratsu, M.; Suzuki, S.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Hosokoshi, Y.; Lan, X.-Z.; Miyazaki, Y.; Inaba, A.; Okada, K. A New Ferrimagnet Based on a Radical-Substituted Radical Cation Salt. J. Am. Chem. Soc. 2009, 131, 4670–4673.
- 14 Zhang, Z.; Wu, Y.-S.; Tang, K.-C.; Chen, C.-L.; Ho, J.-W.; Su, J.; Tian, H.; Chou, P.-T. J. Am. Chem. Soc. 2015, 137, 8509–8520.
- 15 Zhang, Z.; Song, W.; Su, J.; Tian, H. Excited-State Conformational/ Electronic Responses of Saddle-Shaped N,N’-Disubstituted-Dihydrodibenzo[a,c]phenazines: Wide-Tuning Emission from Red to Deep Blue and White Light Combination. Adv. Funct. Mater. 2020, 30, 1902803.
- 16 Chen, W.; Chen, C.-L.; Zhang, Z.; Chen, Y.-A.; Chao, W.-C.; Su, J.; Tian, H.; Chou, P.-T. Snapshotting the Excited-State Planarization of Chemically Locked N,N’-Disubstituted Dihydrodibenzo[a,c]phenazines. J. Am. Chem. Soc. 2017, 139, 1636–1644.
- 17 Zhang, Z.; Chen, C.-L.; Chen, Y.-A.; Wei, Y.-C.; Su, J.; Tian, H.; Chou, P.-T. Tuning the Conformation and Color of Conjugated Polyheterocyclic Skeletons by Installing ortho-Methyl Groups. Angew. Chem. Int. Ed. 2018, 57, 9880–9884.
- 18 Huang, Z.; Jiang, T.; Wang, J.; Ma, X.; Tian, H. Real-Time Visual Monitoring of Kinetically Controlled Self-Assembly. Angew. Chem. Int. Ed. 2021, 60, 2855–2860.
- 19 Zhang, Z.; Sun, G.; Chen, W.; Su, J.; Tian, H. The endeavor of vibration-induced emission (VIE) for dynamic emissions. Chem. Sci. 2020, 11, 7525–7537.
- 20 Zhou, Z.; Chen, D. G.; Saha, M. L.; Wang, H.; Li, X.; Chou, P.-T.; Stang, P. J. Designed Conformation and Fluorescence Properties of Self- Assembled Phenazine-Cored Platinum(II) Metallacycles. J. Am. Chem. Soc. 2019, 141, 5535–5543.
- 21 Qiu, S.; Zhao, Y.; Zhang, L.; Ni, Y.; Wu, Y.; Cong, H.; Qu, D.-H.; Jiang, W.; Wu, J.; Tian, H.; Wang, Z. Axially N-Embedded Quasi-Carbon Nanohoops with Multioxidation States. CCS Chem. 2023, 5, 1763–1772.
- 22 Yang, S.; Zhao, C. X.; Crespi, S.; Mei, J.; Tian, H.; Qu, D.-H. Reversibly modulating a conformation-adaptive fluorophore in [2]catenane. Chem 2021, 7, 1544–1556.
- 23 Chen, W.; Guo, C.; He, Q.; Chi, X.; Lynch, V.; Zhang, Z.; Su, J.; Tian, H.; Sessler, J. L. Molecular Cursor Caliper: A Fluorescent Sensor for Dicarboxylate Dianions. J. Am. Chem. Soc. 2019, 141, 14798–14806.
- 24 Zong, Z.; Zhang, Q.; Qiu, S.-H.; Wang, Q.; Zhao, C.; Zhao, C.-X.; Tian, H.; Qu, D.-H. Dynamic Timing Control over Multicolor Molecular Emission by Temporal Chemical Locking. Angew. Chem. Int. Ed. 2022, 61, e202116414.
- 25 Ma, C.-S.; Yu, C.; Zhao, C.-X.; Zhou, S.-W.; Gu, R. Multicolor emission based on a N,N’-Disubstituted dihydrodibenzo [a,c] phenazine crown ether macrocycle. Front. Chem. 2022, 10, 1087610.
- 26 Yazaki, K.; Noda, S.; Tanaka, Y.; Sei, Y.; Akita, M.; Yoshizawa, M. An M2L4 Molecular Capsule with a Redox Switchable Polyradical Shell. Angew. Chem. Int. Ed. 2016, 55, 15031–15034.
- 27 Jiang, W.-L.; Huang, B.; Zhao, X.-L.; Shi, X.; Yang, H.-B. Strong halide anion binding within the cavity of a conformation-adaptive phenazine-based Pd2L4 cage. Chem 2023, 9, 2655–2668.
- 28 Wu, M.-X.; Hong, Q.-Y.; Li, M.; Jiang, W.-L.; Huang, B.; Lu, S.; Wang, H.; Yang, H.-B.; Zhao, X.-L.; Shi, X. Self-assembly of conformation- adaptive dihydrophenazine-based coordination cages. Chem. Commun. 2024, 60, 1184–1187.
- 29 Barnes, J. C.; Juríček, M.; Strutt, N. L.; Frasconi, M.; Sampath, S.; Giesener, M. A.; McGrier, P. L.; Bruns, C. J.; Stern, C. L.; Sarjeant, A. A.; Stoddart, J. F. ExBox: A Polycyclic Aromatic Hydrocarbon Scavenger. J. Am. Chem. Soc. 2013, 135, 183–192.
- 30 Gong, X.; Young, R. M.; Hartlieb, K. J.; Miller, C.; Wu, Y.; Xiao, H.; Li, P.; Hafezi, N.; Zhou, J.; Ma, L.; Cheng, T.; Goddard, W. A.; Farha, O. K.; Hupp, J. T.; Wasielewski, M. R.; Stoddart, J. F. Intramolecular Energy and Electron Transfer within a Diazaperopyrenium-Based Cyclophane. J. Am. Chem. Soc. 2017, 139, 4107–4116.
- 31 Guo, Q.-H.; Zhou, J.; Mao, H.; Qiu, Y.; Nguyen, M. T.; Feng, Y.; Liang, J.; Shen, D.; Li, P.; Liu, Z.; Wasielewski, M. R.; Stoddart, J. F. TetrazineBox: A Structurally Transformative Toolbox. J. Am. Chem. Soc. 2020, 142, 5419–5428.
- 32 Li, Z.; Han, Y.; Nie, F.; Liu, M.; Zhong, H.; Wang, F. Bright and Robust Phosphorescence Achieved by Non-Covalent Clipping. Angew. Chem. Int. Ed. 2021, 60, 8212–8219.
- 33 Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323.




