Access to Versatile Functionalized Cu(III) Complexes Enabled by Direct Transmetalation to Well-Defined Copper(III) Fluoride Complex Me4N+[Cu(CF3)3F]-
Guangyu Wang
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contribute equally.
Search for more papers by this authorHuaiyuan Li
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contribute equally.
Search for more papers by this authorXuebing Leng
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorLong Lu
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Qilong Shen
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorGuangyu Wang
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contribute equally.
Search for more papers by this authorHuaiyuan Li
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
These authors contribute equally.
Search for more papers by this authorXuebing Leng
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorLong Lu
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Qilong Shen
Key Laboratory of Fluorine and Nitrogen Chemistry and Advanced Materials, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
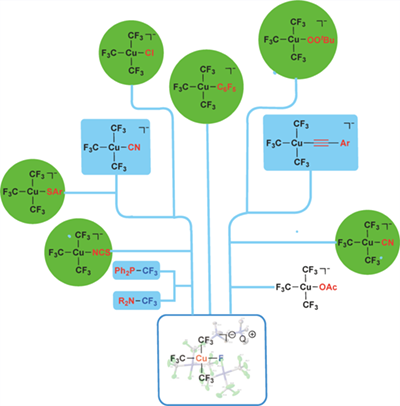
The invention of a well-defined Cu(III) fluoride complex Me4N+[Cu(CF3)3(F)]- 1 enabled to access a versatile of functionalized Cu(III) complexes [Me4N]+[Cu(X)(CF3)3]- (X = C6F5, C6F5C≡C, CN, Cl, N3, tBuOO, SCN, OAc, SAr), many of them for the first time. The availability of these complexes allowed us to evaluate the trans-influence order of ligand in Cu(III) complexes: Bn > CF3– > C6F5– > N3– > py ~ CH3– ~ C6F5C≡C > NO2PhO– ~ tBuOO– ~ CH3COO– > F–.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400041-sup-0001-Supinfo.pdfPDF document, 3.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Doherty, N. M. Transition-Metal Fluoro Compounds Containing Carbonyl, Phosphine, Arsine, And Stibine Ligands. Chem. Rev. 1991, 91, 553–573; (b) Murphy, E. F.; Murugavel, R.; Roesky, H. W. Organometallic Fluorides: Compounds Containing Carbon−Metal−Fluorine Fragments of d-Block Metals. Chem. Rev. 1997, 97, 3425–3468; (c) Nahra, F.; Brill, M.; Gómez-Herrera, A.; Cazin, C. S. J.; Nolan, S. P. Transition Metal Bifluorides. Coord. Chem. Rev. 2016, 307, 65–80.
- 2(a) Benjamin, S. L.; Levason, W.; Reid, G. Medium and High Oxidation State Metal/Non-Metal Fluoride And Oxide–Fluoride Complexes with Neutral Donor Ligands. Chem. Soc. Rev. 2013, 42, 1460–1499; (b) Levason, W.; Monzittu, F. M.; Reid, G. Coordination Chemistry and Applications of Medium/High Oxidation State Metal and Non-Metal Fluoride and Oxide-Fluoride Complexes with Neutral Donor Ligands. Coord. Chem. Rev. 2019, 391, 90–130.
- 3(a) Mondal, P.; Lovisari, M.; Twamley, B.; McDonald, A. R. Fast Hydrocarbon Oxidation by a High-Valent Nickel–Fluoride Complex. Angew. Chem. Int. Ed. 2020, 59, 13044–13050; (b) Lee, H.; Börgel, J.; Ritter, T. Carbon–Fluorine Reductive Elimination from Nickel(III) Complexes. Angew. Chem. Int. Ed. 2017, 56, 6966–6969.
- 4 Meucci, E. A.; Ariafard, A.; Canty, A. J.; Kampf, J. W.; Sanford, M. S. Aryl–Fluoride Bond-Forming Reductive Elimination from Nickel(IV) Centers. J. Am. Chem. Soc. 2019, 141, 13261–13267.
- 5(a) Ball, N. D.; Sanford, M. S. Synthesis and Reactivity of a Mono-σ-Aryl Palladium(IV) Fluoride Complex. J. Am. Chem. Soc. 2009, 131, 3796–3797; (b) Furuya, T.; Benitez, D.; Tkatchouk, E.; Strom, A. E.; Tang, P.; Goddard, III, W. A.; Ritter, T. Mechanism of C−F Reductive Elimination from Palladium(IV) Fluorides. J. Am. Chem. Soc. 2010, 132, 3793–3807; (c) Racowski, J. M.; Gary, J. B.; Sanford, M. S. Carbon(sp3)-Fluorine Bond-Forming Reductive Elimination from Palladium(IV) Complexes. Angew. Chem. Int. Ed. 2012, 51, 3414–3417; (d) Pérez-Temprano, M. H.; Racowski, J. M.; Kampf, J. W.; Sanford. M. S. Competition between sp3-C–N vs sp3-C–F Reductive Elimination from PdIV Complexes. J. Am. Chem. Soc. 2014, 136, 4097–4100.
- 6(a) Mankad, N. P.; Toste, F. D. C(sp3)–F Reductive Elimination from Alkylgold(iii) Fluoride Complexes. Chem. Sci. 2012, 3, 72–76; (b) Kumar, R.; Linden, A.; Nevado, C. Evidence for Direct Transmetalation of AuIII–F with Boronic Acids. J. Am. Chem. Soc. 2016, 138, 13790–13793; (c) Pérez-Bitrián, A.; Baya, M.; Casas, J. M.; Martín, A.; Menjón, B.; Orduna, J. An Organogold(III) Difluoride with a trans Arrangement. Angew. Chem. Int. Ed. 2018, 57, 6517–6521; (d) Ellwanger, M. A.; Steinhauer, S.; Golz, P.; Braun, T.; Riedel, S. Stabilization of Lewis Acidic AuF3 as an N-Heterocyclic Carbene Complex: Preparation and Characterization of [AuF3(SIMes)]. Angew. Chem. Int. Ed. 2018, 57, 7210–7214; (e) Sharp-Buchnall, L.; Barwise, L.; Bennetts, J. D.; Albayer, M.; Dutton, J. L. Reactivity Studies of Cationic Au(III) Difluorides Supported by N Ligands. Organometallics 2020, 39, 3344–3351; (f) Genoux, A.; Bíedrzycki, M.; Merino, E.; Rivera-Chao, E.; Linden, A.; Nevado, C. Synthesis and Characterization of Bidentate (P^N)Gold(III) Fluoride Complexes: Reactivity Platforms for Reductive Elimination Studies. Angew. Chem. Int. Ed. 2021, 60, 4164–4168.
- 7(a) Ye, Y.; Sanford, M. S. Mild Copper-Mediated Fluorination of Aryl Stannanes and Aryl Trifluoroborates. J. Am. Chem. Soc. 2013, 135, 4648–4651; (b) Fier, P. S.; Luo, J.; Hartwig, J. F. Copper-Mediated Fluorination of Arylboronate Esters. Identification of a Copper(III) Fluoride Complex. J. Am. Chem. Soc. 2013, 135, 2552–2559.
- 8(a) Casitas, A.; Canta, M.; Solà, M.; Costas, M.; Ribas, X. Nucleophilic Aryl Fluorination and Aryl Halide Exchange Mediated by a CuI/CuIII Catalytic Cycle. J. Am. Chem. Soc. 2011, 133, 19386–19392; (b) Fier, P. S.; Hartwig, J. F. Copper-Mediated Fluorination of Aryl Iodides. J. Am. Chem. Soc. 2012, 134, 10795–10798; (c) Lee, H. G.; Milner, P. J.; Buchwald, S. L. Pd-Catalyzed Nucleophilic Fluorination of Aryl Bromides. J. Am. Chem. Soc. 2014, 136, 3792–3795; (d) Mu, X.; Zhang, H.; Chen, P.; Liu, G. Copper-Catalyzed Fluorination of 2-Pyridyl Aryl Bromides. Chem. Sci. 2014, 5, 275–280.
- 9(a) Klemm, V. W.; Huss, E. Fluorokomplexe. I. Eisen-, Kobalt-, Nickel- und Kupfer-Komplexe. Zeitschrift Fur Anorganische Chemie 1949, 258, 221–226; (b) Hoppe, R.; Wingefeld, G. Zur Kenntnis der Hexafluorocuprate(III). Z. Anorg. Allg. Chem. 1984, 519, 195–203.
- 10 Bower, J. K.; Cypcar, A. D.; Henriquez, B.; Stieber, S. C. E.; Zhang, S. C(sp3)–H Fluorination with a Copper(II)/(III) Redox Couple. J. Am Chem. Soc. 2020, 142, 8514–8521.
- 11 Willert-Porada, M. A.; Burton, D. J.; Baenziger, N. C. Synthesis and X-ray Structure of Bis(trifluoromethyl)(N,N-diethyldithiocarbamato)- copper; A Remarkably Stable Perfluoroalkylcopper(III) Complex. Chem. Commun. 1989, 1633–1634.
- 12 Naumann, D.; Roy, T.; Tebbe, K.-F.; Crump, W. Synthesis and Structure of Surprisingly Stable Tetrakis(trifluoromethyl)cuprate(III) Salts Angew. Chem. Int. Ed. 1993, 32, 1482–1483.
- 13 Eujen, R.; Hoge, B.; Brauer, D. J. Synthesis and Properties of Donor-Free Bis(difluoromethyl) Cadmium, (CF2H)2Cd NMR Spectroscopic Detection and Structure of Tetrakis(difluoromethyl) Cuprate(III) and Related Compounds. J. Organomet. Chem. 1996, 519, 7–20.
- 14 Romine, A. M.; Nebra, N.; Konovalov, A. I.; Martin, E.; Benet-Buchholz, J.; Grushin, V. V. Easy Access to the Copper(III) Anion [Cu(CF3)4]-. Angew. Chem. Int. Ed. 2015, 54, 2745–2749.
- 15 Tan, X.-Q.; Liu, Z.-L.; Shen, H.-G.; Zhang, P.; Zheng, Z.-Z.; Li, C.-Z. Silver-Catalyzed Decarboxylative Trifluoromethylation of Aliphatic Carboxylic Acids. J. Am. Chem. Soc. 2017, 139, 12430–12433.
- 16(a) Zhang, S.-L.; Bie, W.-F. Ligand-Dependent Formation of Ion-Pair CuI/CuIII Trifluoromethyl Complexes Containing Bisphosphines. Dalton Trans. 2016, 45, 17588–17592; (b) Zhang, S.-L.; Bie, W.-F. Isolation and Characterization of Copper(III) Trifluoromethyl Complexes and Reactivity Studies of Aerobic Trifluoromethylation Of Arylboronic Acids. RSC Adv. 2016, 6, 70902–70906; (c) Zhang, S.-L.; Xiao, C.; Wan, H.-X. Diverse Copper(III) Trifluoromethyl Complexes With Mono-, Bi- and Tridentate Ligands and Their Versatile Reactivity. Dalton Trans. 2018, 47, 4779–4784.
- 17( a) Paeth, M.; Tyndall, S. B.; Chen, L.-Y.; Hong, J.-C.; Carson, W. P.; Liu, X.; Sun, X.; Liu, J.; Yang, K.; Hale, E. M.; Tierney, D. L.; Liu, B.; Cao, Z.; Cheng, M.-J.; Goddard III, W. A.; Liu W. Csp3−Csp3 Bond-Forming Reductive Elimination from Well-Defined Copper(III) Complexes. J. Am. Chem. Soc., 2019, 141, 3153–3159; (b) Yan, W.; Carter, S.; Hsieh, C.-T.; Krause, J. A.; Cheng, M.-J.; Zhang, S.; Liu, W. Copper−Carbon Homolysis Competes with Reductive Elimination in Well-Defined Copper(III) Complexes. J. Am. Chem. Soc. 2023, 145, 26152–26159.
- 18(a) Lu, Z.-H.; Liu, H.; Liu, S.-Y.; Leng, X.-B.; Lan, Y.; Shen, Q. A Key Intermediate in Copper-Mediated Arene Trifluoromethylation, [nBu4N][Cu(Ar)(CF3)3]: Synthesis, Characterization, and C(sp2)-CF3 Reductive Elimination. Angew. Chem. Int. Ed. 2019, 58, 8510–8514; (b) Liu, S.-S.; Liu, H.; Liu, S.-H.; Lu, Z.-H.; Lu, C.-H.; Leng, X.-B.; Lan, Y.; Shen, Q. C(sp3)-CF3 Reductive Elimination from a Five-Coordinate Neutral Copper(III) Complex. J. Am. Chem. Soc. 2020, 142, 9785–9791; (c) Wang, G.-Y.; Li, M.; Leng, X.-B.; Xue, X.-S.; Shen, Q. Neutral Five-Coordinate Arylated Copper(III) Complex: Key Intermediate in Copper-Mediated Arene Trifluoromethylation. Chin. J. Chem. 2022, 40, 1924–1930; (d) Luo, Y.-R.; Li, Y.-L.; Wu, J.; Xue, X.-S.; Hartwig, J. F.; Shen, Q. Oxidative Addition of an Alkyl Halide to Form a Stable Cu(III) Product. Science 2023, 381, 1072–1079.
- 19 Joven-Sancho, D.; Baya, M.; Martín, A.; Orduna, J.; Menjón, B. The First Organosilver(III) Fluoride, [PPh4][(CF3)3AgF]. Chem. Eur. J. 2020, 26, 4471–4475.
- 20 Joven-Sancho, D.; Echeverri, A.; Saffon-Merceron, N.; Contreras-García, J.; Nebra, N. An Organocopper(III) Fluoride Triggering C-CF3 Bond Formation. Angew. Chem. Int. Ed. 2023, e202319412.
- 21 Yang, L.; Powell, D. R.; Houser, R. P. Structural Variation in Copper(i) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, τ4. Dalton Trans. 2007, 955–964.
- 22 Müller, B. G. Fluorides of Copper, Silver, Gold, and Palladium. Angew. Chem. Int. Ed. 1987, 26, 1081–1097.
- 23 Neisen, B. D.; Gagnon, N. L.; Dhar, D.; Spaeth, A. D.; Tolman, W. B. Formally Copper(III)–Alkylperoxo Complexes as Models of Possible Intermediates in Monooxygenase Enzymes. J. Am. Chem. Soc. 2017, 139, 10220–10223.
- 24 Chang, H.-C.; Lo, F.-C.; Liu, W.-C.; Lin, T.-H.; Liaw, W.-F.; Kuo, T.-S.; Lee, W.-Z. Ambient Stable Trigonal Bipyramidal Copper(III) Complexes Equipped with an Exchangeable Axial Ligand. Inorg. Chem. 2015, 54, 5527–5533.
- 25(a) Clark, J. H. Fluoride as a Base in Organic Synthesis. Chem. Rev. 1980, 80, 429–452; (b) Hameed, A.; Alharthy, R. D.; Iqbal, J.; Langer, P. The Role of Naked FluorideIon as Base or catalyst in Organic Synthesis. Tetrahedron 2016, 72, 2763–2812; (c) Tang, L.; Pei, B.-B.; Song, Y.; Xue, F.; Yue, Y.; Feng, C. Synthesis of Mono-Fluoroallenes through Copper-Catalyzed Defluorinative Silylation of α,α-Difluoroalkylalkynes. Chin. J. Chem. 2022, 40, 2035–2039.
- 26 Hartwig, J. F. Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, 2009.
- 27 Liu, H.; Shen, Q. Well-defined Organometallic Copper(III) Complexes: Preparation, Characterization and Reactivity. Coord. Chem. Rev. 2021, 439, 213923.
- 28(a) Mo, X.; Guo, R.; Zhang, G. Recent Development in Copper(I)-Catalyzed Enantioselective Alkynylation Reactions via a Radical Process. Chin. J. Chem. 2023, 41, 481–489; (b) Wang, R.; Lu, L.; Shen, Q. A versatile, Electrophilic Reagent for Monofluoromethylthiolation. Chin. J. Chem. 2023, 41, 2317–2329; (c) Liu, Y.-F.; Ling, Y.-J.; Ge, H.-M.; Lu, L.; Shen, Q. Rational Design and Development of Low-Price, Scalable, Shelf-Stable and Broadly Applicable Electrophilic Sulfonium Ylide- Based Trifluoromethylating Reagents. Chin. J. Chem. 2021, 39, 1667–1682; (d) Si, Y.; Tang, P. Development and Application of Trifluoromethoxylating Reagents. Chin. J. Chem. 2023, 41, 2179–2196.
- 29Deposition numbers 2322820 (for 1), 2322817 (for 3), 2322821 (for 4), 2322815 (for 6), 2322816 (for 7), 2322818 (for 8), 2322819 (for 9), and 2322814 (for 11) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.




