C—F Bond Insertion into Indoles with CHBr2F: An Efficient Method to Synthesize Fluorinated Quinolines and Quinolones
Chao Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorLei Chen
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorHongye Wang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorZixi Yan
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorBin Lyu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorWeiping Lyu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorChangwei Jiang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorDehua Lu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorJiaxing Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorNing Jiao
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Song Song
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
E-mail: [email protected]Search for more papers by this authorChao Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorLei Chen
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorHongye Wang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorZixi Yan
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorBin Lyu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorWeiping Lyu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorChangwei Jiang
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorDehua Lu
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorJiaxing Li
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorNing Jiao
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
Search for more papers by this authorCorresponding Author
Song Song
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Xue Yuan Rd. 38, Beijing, 100191 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
A mild and practical method for synthesizing fluorinated quinoline derivatives, which have a wide range of applications in pharmaceuticals, materials, and organic synthesis, was described through C—F bond insertion into indoles using CHBr2F. The simple conditions, readily availability of CHBr2F, as well as the versatility of the transformations make this strategy very powerful in synthesizing 3-fluoroquinoline and 3-fluoroquinolone. The mechanistic studies reveal that bromofluorocarbene generated in-situ under basic condition was the key intermediate. 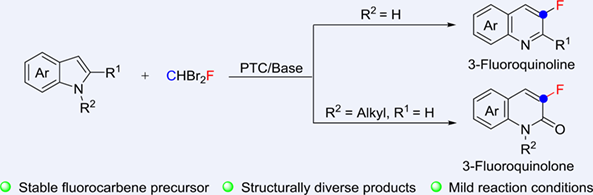
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400033-sup-0001-supinfo.pdfPDF document, 6.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Bçhm, H. J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Muller, K.; Obst-Sander, U.; Stahl, M. Fluorine in Medicinal Chemistry. ChemBioChem 2004, 5, 637–643; (b) Muller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886; (c) Hagmann, T. W. K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369; (d) O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319; (e) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330; (f) Jeschke, P. The Unique Role of Fluorine in the Design of Active Ingredients for Modern Crop Protection. ChemBioChem 2004, 5, 570–589; (g) Li, Y. F. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733; (h) Phelps, M. E. Positron Emission Tomography Provides Molecular Imaging of Biological Processes. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 9226–9233; (i) Ametamey, S. M.; Honer, M.; Schubiger, P. A. Molecular Imaging with PET. Chem. Rev. 2008, 108, 1501–1516.
- 2(a) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359; (b) Meanwell, N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880; (c) Mykhailiuk, P. K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715.
- 3 Morgenthaler, M.; Schweizer, E.; Hoffmann-Roeder, A.; Benini, F.; Martin, R. E.; Jaeschke, G.; Wagner, B.; Fischer, H.; Bendels, S.; Zimmerli, D.; Schneider, J.; Diederich, F.; Kansy, M.; Müller, K. Predicting and Tuning Physicochemical Properties in Lead Optimization: Amine Basicities. ChemMedChem 2007, 2, 1100–1115.
- 4(a) Olsen, J. A.; Banner, D. W.; Seiler, P.; Sander, U. O.; D’Arcy, A.; Stihle, M.; Muller, K.; Diederich, F. A Fluorine Scan of Thrombin Inhibitors to Map the Fluorophilicity/Fluorophobicity of an Enzyme Active Site: Evidence for C-F…C=O Interactions. Angew. Chem. Int. Ed. 2003, 42, 2507–2511; (b) Hof, F.; Scofield, D. M.; Schweizer, W. B.; Diederich, F. A Weak Attractive Interaction between Organic Fluorine and an Amide Group. Angew. Chem. Int. Ed. 2004, 43, 5056–5059.
- 5 Fier, P. S.; Hartwig, J. S. Selective C-H Fluorination of Pyridines and Diazines Inspired by a Classic Amination Reaction. Science 2013, 342, 956–960.
- 6 Li, C.; Yan, Z. X.; Wang, B. D.; Li, J. X.; Lyu, W. P.; Wang, Z. X.; Jiao, N.; Song, S. Regioselective Synthesis of 4-Functionalized Pyridines. Chem 2024, DOI: 10.1016/ j.chempr.2023.10.015.
- 7 Zhang, L.; Yan, J. Y.; Ahmadli, D.; Wang, Z. K.; Ritter, T. Electron-Transfer-Enabled Concerted Nucleophilic Fluorination of Azaarenes: Selective C–H Fluorination of Quinolines. J. Am. Chem. Soc. 2023, 145, 20182–20188.
- 8(a) Sather, C.; Buchwald, S. L. The Evolution of Pd0/PdII-Catalyzed Aromatic Fluorination. Acc. Chem. Res. 2016, 49, 2146–2157; (b) Chen, T. Q.; Pedersen, P. S.; Dow, N. W.; Fayad, R.; Hauke, C. E.; Rosko, M. C.; Danilov, E. O.; Blakemore, D. C.; Dechert-Schmitt, A. M.; Knauber, T.; Castellano, F. N.; MacMillan, D. W. C. A Unified Approach to Decarboxylative Halogenation of (Hetero)aryl Carboxylic Acids. J. Am. Chem. Soc. 2022, 144, 8296–8305; (c) Taylor, N. J.; Emer, E.; Preshlock, S.; Schedler, M.; Tredwell, M.; Verhoog, S.; Mercier, J.; Genicot, C.; Gouverneur, V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276.
- 9(a) Vitaku, E.; Smith, D. T.; Njardarson, J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274; (b) Zhu, M.; Zhang, X.; Zheng, C.; You, S. L. Energy-Transfer-Enabled Dearomative Cycloaddition Reactions of Indoles/Pyrroles via Excited-State Aromatics. Acc. Chem. Res. 2022, 55, 2510–2525; (c) Zhang, Y. C.; Jiang, F.; Shi, F. Organocatalytic Asymmetric Synthesis of Indole-Based Chiral Heterocycles: Strategies, Reactions, and Outreach. Acc. Chem. Res. 2020, 53, 425–446.
- 10(a) Wen J.; Shi, Z. Z. From C4 to C7: Innovative Strategies for Site-Selective Functionalization of Indole C–H Bonds. Acc. Chem. Res. 2021, 54, 1723–1736; (b) Prabagar, B.; Yang Y.; Shi, Z. Z. Site-Selective C–H Functionalization to Access the Arene Backbone of Indoles and Quinolines. Chem. Soc. Rev. 2021, 50, 11249–11269; (c) Meucci, E. A.; Nguyen, S. N.; Camasso, N. M.; Chong, E.; Ariafard, A.; Canty, A. J.; Sanford, M. S. Nickel(IV)-Catalyzed C–H Trifluoromethylation of (Hetero)arenes. J. Am. Chem. Soc. 2019, 141, 12872–12879; (d) Wang, Z. J.; Chen, X.; Wu, L.; Wong, J. J.; Liang, Y.; Zhao, Y.; Houk, K. N.; Shi, Z. Z. Metal-Free Directed C−H Borylation of Pyrroles. Angew. Chem. Int. Ed. 2021, 60, 8500–8504; (e) Schweitzer-Chaput, B.; Horwitz, M. A.; de Pedro Beato, E.; Melchiorre, P. Photochemical Generation of Radicals from Alkyl Electrophiles Using a Nucleophilic Organic Catalyst. Nat. Chem. 2019, 11, 129–135; (f) Lv, J. H.; Chen, X. Y.; Xue, X. S.; Zhao, B. l.; Liang, Y.; Wang, M. Y.; Jin, L. Q.; Yuan, Y.; Han, Y.; Zhao, Y.; Lu, Y.; Zhao, J.; Sun, W. Y.; Houk, K. N.; Shi, Z. Z. Metal-free Directed sp2-C–H Borylation. Nature 2019, 575, 336–340.
- 11(a) Jurczyk, J.; Woo, J.; Kim, S. F.; Dherange, B. D.; Sarpong, R.; Levin, M. D. Single-atom Logic for Heterocycle Editing. Nat. Synth. 2022, 1, 352–364;
(b) Joynson, B. W.; Ball, L. T. Skeletal Editing: Interconversion of Arenes and Heteroarenes. Helv. Chim. Acta 2023, 106, e202200182;
(c) Liu, Z.; Sivaguru, P.; Ning, Y.; Wu, Y.; Bi, X. Skeletal Editing of (Hetero)Arenes Using Carbenes. Chem. Eur. J. 2023, 29, e202301227;
(d) Ciamician, G. L.; Dennstedt, M. Ueber die Einwirkung des Chloroforms auf die Kaliumverbindung Pyrrols. Ber. Dtsch. Chem. Ges. 1881, 14, 1153;
10.1002/cber.188101401240 Google Scholar(e) Mortén, M.; Hennum, M.; Bonge-Hansen, T. Synthesis of Quinoline-3-Carboxylates by a Rh(II)-Catalyzed Cyclopropanation-ring Expansion Reaction of Indoles with Halodiazoacetates. Beilstein J. Org. Chem. 2015, 11, 1944–1949; (f) Reisenbauer, J. C.; Green, O.; Franchino, A.; Finkelstein, P.; Morandi, B. Late-Stage Diversification of Indole Skeletons through Nitrogen Atom Insertion. Science 2022, 377, 1104–1109; (g) Dherange, B. D.; Kelly, P. Q.; Liles, J. P.; Sigman, M. S.; Levin, M. D. Carbon Atom Insertion into Pyrroles and Indoles Promoted by Chlorodiazirines. J. Am. Chem. Soc. 2021, 143, 11337–11344; (h) Lin, Z.; Ji, J.; Zhou, S. X.; Zhang, F.; Wu, J. Q.; Guo, Y. L.; Liu, W. Processing 2-Methyl-l-Tryptophan through Tandem Transamination and Selective Oxygenation Initiates Indole Ring Expansion in the Biosynthesis of Thiostrepton. J. Am. Chem. Soc. 2017, 139, 12105–12108; (i) Joynson, B. W.; Cumming, G. R.; Ball, L. T. Photochemically Mediated Ring Expansion of Indoles and Pyrroles with Chlorodiazirines: Synthetic Methodology and Thermal Hazard Assessment. Angew. Chem. Int. Ed. 2023, 62, e202305081; (j) Cao, W. B.; Li, S. J.; Xu, M. M.; Li, H. Y.; Xu, X. P.; Lan, Y.; Ji, S. J. Hydrogen-Bonding-Promoted Cascade Rearrangement Involving the Enlargement of Two Rings: Efficient Access to Polycyclic Quinoline Derivatives. Angew. Chem. Int. Ed. 2020, 59, 21425–21430; (K) Aksenov, A. V.; Smirnov, A. N.; Aksenov, N. A.; Aksenova, I. V.; Frolova, L. V.; Kornienko, A.; Magedovzb, I. V.; Rubin, M. Metal-Free Transannulation Reaction of Indoles with Nitrostyrenes: A Simple Practical Synthesis of 3-Substituted 2-Quinolones. Chem. Commun. 2013, 49, 9305–9307.
- 12During the preparation of our manuscript, Xu and coworkers reported similar work about one-carbon ring expansion of indoles. Guo, H. X.; Qiu, S. Q.; Xu, P. One-Carbon Ring Expansion of Indoles and Pyrroles: A Straightforward Access to 3-Fluorinated Quinolines and Pyridines. Angew. Chem. Int. Ed. 2023, e202317104.
- 13(a) Brahms, D. L. S.; Dailey, W. P. Fluorinated Carbenes. Chem. Rev. 1996, 96, 1585–1632; (b) Bychek, R.; Mykhailiuk, P. K. A Practical and Scalable Approach to Fluoro-Substituted Bicyclo[1.1.1]pentanes. Angew. Chem. Int. Ed. 2022, 61, e202205103; (c) Yue, W. J.; Martin, R. Ni-Catalyzed Site-Selective Hydrofluoroalkylation of Terminal and Internal Olefins. ACS Catal. 2022, 12, 12132–12137; (d) Chen, F.; Xu, X. H.; Qing, F. L. Photoredox-Catalyzed Addition of Dibromofluoromethane to Alkenes: Direct Synthesis of 1-Bromo-1-fluoroalkanes. Org. Lett. 2021, 23, 2364–2369; (e) Hell, S. M.; Meyer, C. F.; Ortalli, S.; Sap, J. B. I.; Chen, X. X.; Gouverneur, V. Hydrofluoromethylation of Alkenes with Fluoroiodomethane and Beyond. Chem. Sci. 2021, 12, 12149–12155.
- 14(a) Kraus, J. M.; Verlinde, C. L. M. J.; Karimi, M.; Lepesheva, G. I.; Gelb, M. H.; Buckner, F. S. Rational Modification of a Candidate Cancer Drug for Use Against Chagas Disease. J. Med. Chem. 2009, 52, 1639–1647; (b) Claassen, G.; Brin, E.; Crogan-Grundy, C.; Vaillancourt, M. T.; Zhang, H. Z.; Cai, S. X.; Drewe, J.; Tseng, B.; Kasibhatla, S. Selective Activation of Apoptosis by A Novel Set of 4-aryl-3-(3-aryl-1-oxo-2- propenyl)-2(1H)-quinolinones through a Myc-dependent Pathway. Cancer Lett. 2009, 274, 243–249; (c) Cheng, P.; Zhang, Q.; Ma, Y. B.; Jiang, Z. Y.; Zhang, X. M.; Zhang, F. X.; Chen, J. J. Synthesis and In Vitro Anti-hepatitis B Virus Activities of 4-aryl-6-chloro-quinolin-2- one and 5-aryl-7-chloro-1,4-benzodiazepine Derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 3787–3789; (d) Poulie, C. B. M.; Bunch, L. Heterocycles as Nonclassical Bioisosteres of α-Amino Acids. ChemMedChem 2013, 8, 205–215; (e) Sabbah, D. A.; Simms, N. A.; Wang, W.; Dong, Y.; Ezell, E. L.; Brattain, M. G.; Vennerstrom, J. L.; Zhong, H. A. N-Phenyl-4-hydroxy-2-quinolone-3-carboxamides as Selective Inhibitors of Mutant H1047R Phosphoinositide-3-kinase (PI3Kα). Bioorg. Med. Chem. 2012, 20, 7175–7183.
- 15 Rueping, M.; Theissmann, T.; Antonchick, A. P. Metal-Free Brønsted Acid Catalyzed Transfer Hydrogenation - New Organocatalytic Reduction of Quinolines. Synlett 2006, 7, 1071–1074.
- 16 Roudesly, F.; Veiros, L. F.; Oble, J.; Poli, G. Pd-Catalyzed Direct C–H Alkenylation and Allylation of Azine N-Oxides. Org. Lett. 2018, 20, 2346–2350.
- 17 Rees, C. W.; Smithen, C. E. The mechanism of Heterocyclic Ring Expansions. Part I. The Reaction of 2,3-Dimethylindole with Dichlorocarbene. J. Chem. Soc. 1964, 928–937.
- 18 Closs, G.; Schwartz, G. Notes- Ring-Expansion of Pyrrole and Indole. J. Org. Chem. 1961, 26, 2609.
- 19 Dutta, R.; Mandal, D.; Panda, N.; Mondal, N. B.; Banerjee, S.; Kumar, S.; Weber, M.; Lugerb, P.; Sahu, N. P. General Methodology for Synthesis of Fused Tricyclic Oxazino-2-quinolones under Phase-transfer Catalyzed Conditions. Tetrahedron Lett. 2004, 45, 9361–9364.




