Stable Electron Spin Pan on Aromatic Oxalic Acid Radical
Jiaxing Huang
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorChenghui Liao
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorLongtian Guan
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorQiao Meng
Faculty of Materials Science, MSU-BIT University, Shenzhen, Guangdong, 518172 China
Search for more papers by this authorSichen Gu
Faculty of Materials Science, MSU-BIT University, Shenzhen, Guangdong, 518172 China
Search for more papers by this authorZhicai He
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorCorresponding Author
Yuan Li
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
E-mail: [email protected]Search for more papers by this authorJiaxing Huang
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorChenghui Liao
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorLongtian Guan
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorQiao Meng
Faculty of Materials Science, MSU-BIT University, Shenzhen, Guangdong, 518172 China
Search for more papers by this authorSichen Gu
Faculty of Materials Science, MSU-BIT University, Shenzhen, Guangdong, 518172 China
Search for more papers by this authorZhicai He
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
Search for more papers by this authorCorresponding Author
Yuan Li
State Key Laboratory of Luminescent Materials and Devices, Institute of Polymer Optoelectronic Materials and Devices, South China University of Technology, Guangzhou, Guangdong, 510640 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
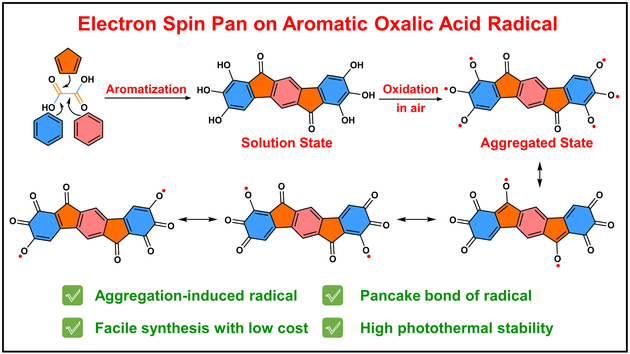
The stability of organic radicals in ambient condition is important for their practical application. During the development of organic radical chemistry, the electron-withdrawing and steric hindrance groups are usually introduced to improve the stability of radicals via reducing the reactivity of radicals with oxygen in air. Herein, the electron-withdrawing carbonyl groups are introduced to construct a planar aromatic oxalic acid radical (IDF-O8) with two-dimensional electron spin pan structure. Interestingly, IDF-O8 exhibited a low optical bandgap of 0.91 eV in film, however, the multiple quinone resonance structures between electron-withdrawing ketone and phenol radicals contribute to the high stability of open-shell radical IDF-O8 without protection of large steric hindrance groups. Under the irradiation of 808 nm (1.2 W·cm–2), IDF-O8 reaches 147 °C in powder state. This work provides an efficient synthesis route for the open-shell electron spin pan system, which is different from the famous fullerene, carbon nanotube and graphene. The electron spin pan can be extended to spin tube or spin sphere system based on the design strategy of aromatic inorganic acid radicals in future.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400001-sup-0001-supinfo.pdfPDF document, 2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Tang, H.; Liang, Y.; Liu, C.; Hu, Z.; Deng, Y.; Guo, H.; Yu, Z.; Song, A.; Zhao, H.; Zhao, D.; Zhang, Y.; Guo, X.; Pei, J.; Ma, Y.; Cao, Y.; Huang, F. A solution-processed n-type conducting polymer with ultrahigh conductivity. Nature 2022, 611, 271–277.
- 2
Yang, C.; Chen, Z.; Yu, C.; Cao, J.; Ke, G.; Zhu, W.; Liang, W.; Huang, J.; Cai, W.; Saha, C.; Sabuj, M. A.; Rai, N.; Li, X.; Yang, J.; Li, Y.; Huang, F.; Guo, X. Regulation of quantum spin conversions in a single molecular radical. Nat. Nanotechnol. 2024, DOI: https://doi.org/10.1038/s41565-024-01632-2.
10.1038/s41565-024-01632-2 Google Scholar
- 3 Lu, C.; Cho, E.; Wan, K.; Wu, C.; Gao, Y.; Coropceanu, V.; Brédas, J.-L.; Li, F. Achieving Nearly 100% Photoluminescence Quantum Efficiency in Organic Radical Emitters by Fine-Tuning the Effective Donor-Acceptor Distance. Adv. Funct. Mater. 2024, 2314811.
- 4 Ratera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular materials. Chem. Soc. Rev. 2012, 41, 303–349.
- 5 Hicks, R. G. What's new in stable radical chemistry? Org. Biomol. Chem. 2007, 5, 1321–1338.
- 6 Ariai, J.; Ziegler, M.; Würtele, C.; Gellrich, U. An N-Heterocyclic Quinodimethane: A Strong Organic Lewis Base Exhibiting Diradical Reactivity. Angew. Chem. Int. Ed. 2024, 63, e202316720.
- 7 Ai, X.; Evans, E. W.; Dong, S.; Gillett, A. J.; Guo, H.; Chen, Y.; Hele, T. J. H.; Friend, R. H.; Li, F. Efficient radical-based light-emitting diodes with doublet emission. Nature 2018, 563, 536–540.
- 8
Song, S.; Pinar Solé, A.; Matěj, A.; Li, G.; Stetsovych, O.; Soler, D.; Yang, H.; Telychko, M.; Li, J.; Kumar, M.; Chen, Q.; Edalatmanesh, S.; Brabec, J.; Veis, L.; Wu, J.; Jelinek, P.; Lu, J. Highly entangled polyradical nanographene with coexisting strong correlation and topological frustration. Nat. Chem. 2024, DOI: https://doi.org/10.1038/s41557-024-01453-9.
10.1038/s41557-024-01453-9 Google Scholar
- 9 Wu, S.; Han, Y.; Ni, Y.; Hou, X.; Wei, H.; Li, Z.; Wu, J. Unveiling Möbius/Hückel Topology and Aromaticity in A Core-Expanded [10]Annulene at Different Oxidation States. Angew. Chem. Int. Ed. 2024, 63, e202320144.
- 10 Kawai, S.; Silveira, O. J.; Kurki, L.; Yuan, Z.; Nishiuchi, T.; Kodama, T.; Sun, K.; Custance, O.; Lado, J. L.; Kubo, T.; Foster, A. S. Local probe-induced structural isomerization in a one-dimensional molecular array. Nat. Commun. 2023, 14, 7741.
- 11 Nishiuchi, T.; Aibara, S.; Kubo, T. Synthesis and Properties of a Highly Congested Tri(9-anthryl)methyl Radical. Angew. Chem. Int. Ed. 2018, 57, 16516–16519.
- 12 Zhang, D.; Liu, C.; Zhang, K.; Jia, Y.; Zhong, W.; Qiu, W.; Li, Y.; Heumüller, T.; Forberich, K.; Le Corre, V. M.; Lüer, L.; Li, N.; Huang, F.; Brabec, C. J.; Ying, L. Observation of reversible light degradation in organic photovoltaics induced by long-persistent radicals. Energy Environ. Sci. 2023, 16, 5339–5349.
- 13 Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 2011, 40, 3336–3355.
- 14 Janoschka, T.; Hager, M. D.; Schubert, U. S. Powering up the Future: Radical Polymers for Battery Applications. Adv. Mater. 2012, 24, 6397–6409.
- 15 Minami, T.; Nakano, M. Diradical Character View of Singlet Fission. J. Phys. Chem. Lett. 2012, 3, 145–150.
- 16 Peng, Q.; Obolda, A.; Zhang, M.; Li, F. Organic Light-Emitting Diodes Using a Neutral π Radical as Emitter: The Emission from a Doublet. Angew. Chem. Int. Ed. 2015, 54, 7091–7095.
- 17 Zhang, M.; Li, T.; Liu, X.; Zhang, C.; Li, X. Molecular Revealing the High-stable Polycyclic Azine Derivatives for Long-Lifetime Aqueous Organic Flow Batteries. Adv. Funct. Mater. 2023, 34, 2312608.
- 18 Tang, C.; Song, L.; Zhou, K.; Ren, P.; Zhao, E.; He, Z. Manipulating D–A interaction to achieve stable photoinduced organic radicals in triphenylphosphine crystals. Chem. Sci. 2023, 14, 1871–1877.
- 19 Zhang, S.; Ma, L.; Ma, W.; Chen, L.; Gao, K.; Yu, S.; Zhang, M.; Zhang, L.; He, G. Selenoviologen-Appendant Metallacycles with Highly Stable Radical Cations and Long-Lived Charge Separation States for Electrochromism and Photocatalysis. Angew. Chem. Int. Ed. 2022, 61, e202209054.
- 20 Gu, J.; Wu, W.; Danovich, D.; Hoffmann, R.; Tsuji, Y.; Shaik, S. Valence Bond Theory Reveals Hidden Delocalized Diradical Character of Polyenes. J. Am. Chem. Soc. 2017, 139, 9302–9316.
- 21 Johansson, K. O.; Head-Gordon, M. P.; Schrader, P. E.; Wilson, K. R.; Michelsen, H. A. Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth. Science 2018, 361, 997–1000.
- 22 Hu, C.; Kuhn, L.; Makurvet, F. D.; Knorr, E. S.; Lin, X.; Kawade, R. K.; Mentink-Vigier, F.; Hanson, K.; Alabugin, I. V. Tethering Three Radical Cascades for Controlled Termination of Radical Alkyne peri-Annulations: Making Phenalenyl Ketones without Oxidants. J. Am. Chem. Soc. 2024, 146, 4187–4211.
- 23 Li, G.; Han, Y.; Zou, Y.; Lee, J. J. C.; Ni, Y.; Wu, J. Dearomatization Approach Toward a Superbenzoquinone-Based Diradicaloid, Tetraradicaloid, and Hexaradicaloid. Angew. Chem. Int. Ed. 2019, 58, 14319–14326.
- 24 Li, G.; Phan, H.; Herng, T. S.; Gopalakrishna, T. Y.; Liu, C.; Zeng, W.; Ding, J.; Wu, J. Toward Stable Superbenzoquinone Diradicaloids. Angew. Chem. Int. Ed. 2017, 56, 5012–5016.
- 25 Koppens, F. H. L.; Mueller, T.; Avouris, P.; Ferrari, A. C.; Vitiello, M. S.; Polini, M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat. Nanotechnol. 2014, 9, 780–793.
- 26 Cao, Y.; Fatemi, V.; Demir, A.; Fang, S.; Tomarken, S. L.; Luo, J. Y.; Sanchez-Yamagishi, J. D.; Watanabe, K.; Taniguchi, T.; Kaxiras, E.; Ashoori, R. C.; Jarillo-Herrero, P. Correlated insulator behaviour at half-filling in magic-angle graphene superlattices. Nature 2018, 556, 80–84.
- 27 Graham, K. R.; Cabanetos, C.; Jahnke, J. P.; Idso, M. N.; El Labban, A.; Ngongang Ndjawa, G. O.; Heumueller, T.; Vandewal, K.; Salleo, A.; Chmelka, B. F.; Amassian, A.; Beaujuge, P. M.; McGehee, M. D. Importance of the Donor:Fullerene Intermolecular Arrangement for High-Efficiency Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 9608–9618.
- 28 Bai, Y.; Dong, Q.; Shao, Y.; Deng, Y.; Wang, Q.; Shen, L.; Wang, D.; Wei, W.; Huang, J. Enhancing stability and efficiency of perovskite solar cells with crosslinkable silane-functionalized and doped fullerene. Nat. Commun. 2016, 7, 12806.
- 29 Chung, H. T.; Won, J. H.; Zelenay, P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat. Commun. 2013, 4, 1922.
- 30 De Volder, M. F. L.; Tawfick, S. H.; Baughman, R. H.; Hart, A. J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539.
- 31 Dai, X.; Wang, Y.; Sun, X.; Li, K.; Pan, J.; Wang, J.; Zhuang, T.; Chong, D.; Yan, J.; Wang, H. All-Automated Fabrication of Free-Standing and Scalable Photo Thermoelectric Devices with High Performance. Adv. Mater. 2024, 2312570.
- 32 Wang, H.; Li, C.; Zhang, J.; Yang, Z.; Li, J.; Cao, Y.; Wu, K.; Liu, Z.; Hao, J.; Ye, X. NIR-Excitable POM-Encapsulated Yb-Bi2S3 Decorated Graphene for Wearable Photoelectrochemical Sensing. Adv. Funct. Mater. 2024, 2315917.
- 33 Komeily Nia, Z.; Chen, J.-Y.; Tang, B.; Yuan, B.; Wang, X.-G.; Li, J.-L. Optimizing the free radical content of graphene oxide by controlling its reduction. Carbon 2017, 116, 703–712.
- 34 Li, L.; Li, Y. A Study on the Origin of the Radical in Fullerene and Graphene. J. Phys. Chem. C 2018, 122, 8780–8787.
- 35 Li, X.; Wang, J.; Duan, X.; Li, Y.; Fan, X.; Zhang, G.; Zhang, F.; Peng, W. Fine-Tuning Radical/Nonradical Pathways on Graphene by Porous Engineering and Doping Strategies. ACS Catal. 2021, 11, 4848–4861.
- 36 Hong, J.; Kim, J.; Bae, J.-H.; Jin, H.; Lee, S. K.; Lee, K. H.; Lee, Y.-S.; Chun, D. W. Revealing the Hidden Role of Radical Scavengers: Unraveling the Key to Tailoring the Formation of the hcp PdHx Phase in Graphene Liquid Cells. Adv. Funct. Mater. 2024, 2311293.
- 37 Li, X.; Cao, J.; Chen, J.; Zhu, Y.; Xia, H.; Xu, Z.; Gu, C.; Xie, J.; Jones, M.; Lyu, C.; Corbin, J.; Li, X.; Hu, W. UV-Induced Synthesis of Graphene Supported Iridium Catalyst with Multiple Active Sites for Overall Water Splitting. Adv. Funct. Mater. 2024, 2313530.
- 38 Chen, C.; Liang, Q.; Chen, Z.; Zhu, W.; Wang, Z.; Li, Y.; Wu, X.; Xiong, X. Phenoxy Radical-Induced Formation of Dual-Layered Protection Film for High-Rate and Dendrite-Free Lithium-Metal Anodes. Angew. Chem. Int. Ed. 2021, 60, 26718–26724.
- 39
Huang, J.; Wang, Z.; Zhu, W.; Li, Y. J. A. Solution-processed D-A-π-A-D radicals for highly efficient photothermal conversion. Aggregate 2023, 5, e426.
10.1002/agt2.426 Google Scholar
- 40
Wang, Z.; Zhou, J.; Zhang, Y.; Zhu, W.; Li, Y. Accessing Highly Efficient Photothermal Conversion with Stable Open-Shell Aromatic Nitric Acid Radicals. Angew. Chem. Int. Ed. 2022, 134, e202113653.
10.1002/ange.202113653 Google Scholar
- 41 Zhou, J.; Zhu, W.; Zeng, M.; Yang, Q.; Li, P.; Lan, L.; Peng, J.; Li, Y.; Huang, F.; Cao, Y. Aromatic inorganic acid radical. Sci. China Chem. 2019, 62, 1656–1665.
- 42 Chen, Z.; Li, Y.; Huang, F. J. C. Persistent and stable organic radicals: Design, synthesis, and applications. Chem 2021, 7, 288–332.
- 43 Zhu, W.-H. A new strategy enabling intramolecular motion to obtain advanced photothermal materials. Sci. China Chem. 2019, 62, 659–661.
- 44 Li, J.; Ou, H.; Li, J.; Yang, X.; Ge, C.; Ding, D.; Gao, X. Large π-extended donor-acceptor polymers for highly efficient in vivo near-infrared photoacoustic imaging and photothermal tumor therapy. Sci. China Chem. 2021, 64, 2180–2192.
- 45 East, N. R.; Naumann, R.; Förster, C.; Ramanan, C.; Diezemann, G.; Heinze, K. Oxidative two-state photoreactivity of a manganese(IV) complex using near-infrared light. Nat. Chem. 2024, DOI: https://doi.org/10.1038/s41557-024-01446-8.
- 46 Song, Y.; Zhan, G.; Zhou, S.-F. Design of Near Infrared Light-Powered Copper Phyllosilicate Nanomotors for Cuproptosis-Based Synergistic Cancer Therapy. Adv. Funct. Mater. 2024, 2314568.
- 47 Zeng, Z.; Ishida, M.; Zafra, J. L.; Zhu, X.; Sung, Y. M.; Bao, N.; Webster, R. D.; Lee, B. S.; Li, R.-W.; Zeng, W.; et al. Pushing extended p-quinodimethanes to the limit: stable tetracyano-oligo (N-annulated perylene) quinodimethanes with tunable ground states. J. Am. Chem. Soc. 2013, 135, 6363–6371.
- 48 Yuan, D.; Huang, D.; Rivero, S. M.; Carreras, A.; Zhang, C.; Zou, Y.; Jiao, X.; McNeill, C. R.; Zhu, X.; Di, C.-a.; et al. Cholesteric aggregation at the quinoidal-to-diradical border enabled stable n-doped conductor. Chem 2019, 5, 964–976.
- 49 Zhang, C.; Medina Rivero, S.; Liu, W.; Casanova, D.; Zhu, X.; Casado, J. Stable Cross-Conjugated Tetrathiophene Diradical. Angew. Chem. Int. Ed. 2019, 58, 11291–11295.
- 50 Takahashi, T.; Matsuoka, K.-i.; Takimiya, K.; Otsubo, T.; Aso, Y. Extensive quinoidal oligothiophenes with dicyanomethylene groups at terminal positions as highly amphoteric redox molecules. J. Am. Chem. Soc. 2005, 127, 8928–8929.
- 51 Lawrence, J.; He, Y.; Wei, H.; Su, J.; Song, S.; Wania Rodrigues, A.; Miravet, D.; Hawrylak, P.; Zhao, J.; Wu, J.; Lu, J. Topological Design and Synthesis of High-Spin Aza-triangulenes without Jahn–Teller Distortions. ACS Nano 2023, 17, 20237–20245.
- 52 Sun, Z.; Ye, Q.; Chi, C.; Wu, J. Low band gap polycyclic hydrocarbons: from closed-shell near infrared dyes and semiconductors to open-shell radicals. Chem. Soc. Rev. 2012, 41, 7857–7889.
- 53 Zeng, W.; Wu, J. Open-Shell Graphene Fragments. Chem 2021, 7, 358–386.
- 54 Li, Y.; Heng, W.-K.; Lee, B. S.; Aratani, N.; Zafra, J. L.; Bao, N.; Lee, R.; Sung, Y. M.; Sun, Z.; Huang, K.-W.; Webster, R. D.; López Navarrete, J. T.; Kim, D.; Osuka, A.; Casado, J.; Ding, J.; Wu, J. Kinetically Blocked Stable Heptazethrene and Octazethrene: Closed-Shell or Open-Shell in the Ground State? J. Am. Chem. Soc. 2012, 134, 14913–14922.
- 55 Chen, Z.; Li, W.; Zhang, Y.; Wang, Z.; Zhu, W.; Zeng, M.; Li, Y. Aggregation-Induced Radical of Donor–Acceptor Organic Semiconductors. J. Phys. Chem. Lett. 2021, 12, 9783–9790.
- 56 Chen, Z.; Li, W.; Sabuj, M. A.; Li, Y.; Zhu, W.; Zeng, M.; Sarap, C. S.; Huda, M. M.; Qiao, X.; Peng, X.; et al. Evolution of the electronic structure in open-shell donor-acceptor organic semiconductors. Nat. Commun. 2021, 12, 5889.
- 57 Xue, Y.; Guo, P.; Yip, H.-L.; Li, Y.; Cao, Y. General design of self-doped small molecules as efficient hole extraction materials for polymer solar cells. J. Mater. Chem. A 2017, 5, 3780–3785.
- 58 Xia, X.; Wang, R.; Hu, Y.; Yao, Q.; Long, S.; Sun, W.; Fan, J.; Peng, X. A sulfur-substituted hemicyanine for cancer photothermal therapy without influence of intracellular viscosity. Sci. China Chem. 2022, 65, 821–828.
- 59 Ren, F.; Li, Z.; Li, K.; Zheng, X.; Shi, J.; Zhang, C.; Guo, H.; Tong, B.; Xi, L.; Cai, Z.; Dong, Y. Donor strategy for promoting nonradiative decay to achieve an efficient photothermal therapy for treating cancer. Sci. China Chem. 2021, 64, 1530–1539.
- 60 Li, G.; Yue, Q.; Fu, P.; Wang, K.; Zhou, Y.; Wang, J. Ionic Dye Based Covalent Organic Frameworks for Photothermal Water Evaporation. Adv. Funct. Mater. 2023, 33, 2213810.
- 61 Zeng, Z.; Shi, X.; Chi, C.; López Navarrete, J. T.; Casado, J.; Wu, J. Pro-aromatic and anti-aromatic π-conjugated molecules: an irresistible wish to be diradicals. Chem. Soc. Rev. 2015, 44, 6578–6596.
- 62 Mayer, K. S.; Adams, D. J.; Eedugurala, N.; Lockart, M. M.; Mahalingavelar, P.; Huang, L.; Galuska, L. A.; King, E. R.; Gu, X.; Bowman, M. K.; Azoulay, J. D. Topology and ground state control in open-shell donor-acceptor conjugated polymers. Cell Rep. Phys. Sci. 2021, 2, 100467.
- 63 London, A. E.; Chen, H.; Sabuj, M. A.; Tropp, J.; Saghayezhian, M.; Eedugurala, N.; Zhang, B. A.; Liu, Y.; Gu, X.; Wong, B. M.; Rai, N.; Bowman, M. K.; Azoulay, J. D. A high-spin ground-state donor-acceptor conjugated polymer. Sci. Adv. 2019, 5, eaav2336.
- 64 Joo, Y.; Huang, L.; Eedugurala, N.; London, A. E.; Kumar, A.; Wong, B. M.; Boudouris, B. W.; Azoulay, J. D. Thermoelectric Performance of an Open-Shell Donor–Acceptor Conjugated Polymer Doped with a Radical-Containing Small Molecule. Macromolecules 2018, 51, 3886–3894.
- 65 Garner, L. E.; Viswanathan, V. N.; Arias, D. H.; Brook, C. P.; Christensen, S. T.; Ferguson, A. J.; Kopidakis, N.; Larson, B. W.; Owczarczyk, Z. R.; Pfeilsticker, J. R.; et al. Photobleaching dynamics in small molecule vs. polymer organic photovoltaic blends with 1,7-bis-trifluoromethylfullerene. J. Mater. Chem. A 2018, 6, 4623–4628.
- 66 Chan, W. C.; Nie, S. J. S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018.
- 67 Shuku, Y.; Mizuno, A.; Ushiroguchi, R.; Hyun, C. S.; Ryu, Y. J.; An, B.-K.; Kwon, J. E.; Park, S. Y.; Tsuchiizu, M.; Awaga, K. An exotic band structure of a supramolecular honeycomb lattice formed by a pancake π–π interaction between triradical trianions of triptycene tribenzoquinone. Chem. Commun. 2018, 54, 3815–3818.
- 68 Kubo, T.; Shimizu, A.; Sakamoto, M.; Uruichi, M.; Yakushi, K.; Nakano, M.; Shiomi, D.; Sato, K.; Takui, T.; Morita, Y.; Nakasuji, K. Synthesis, Intermolecular Interaction, and Semiconductive Behavior of a Delocalized Singlet Biradical Hydrocarbon. Angew. Chem. Int. Ed. 2005, 44, 6564–6568.
- 69 Liu, Y.; Liu, B.; Ma, C.-Q.; Huang, F.; Feng, G.; Chen, H.; Hou, J.; Yan, L.; Wei, Q.; Luo, Q.; Bao, Q.; Ma, W.; Liu, W.; Li, W.; Wan, X.; Hu, X.; Han, Y.; Li, Y.; Zhou, Y.; Zou, Y.; Chen, Y.; Liu, Y.; Meng, L.; Li, Y.; Chen, Y.; Tang, Z.; Hu, Z.; Zhang, Z.-G.; Bo, Z. Recent progress in organic solar cells (Part II device engineering). Sci. China Chem. 2022, 65, 1457–1497.
- 70 Wu, F.; Sun, Y.; Gao, H.; Zhi, X.; Zhao, Y.; Shen, Z. Boosting near-infrared photothermal/photoacoustic conversion performance of anthracene-fused porphyrin via paramagnetic ion coordination strategy. Sci. China Chem. 2023, 66, 164–173.
- 71 Wu, S.; Wang, W.; Li, M.; Cao, L.; Lyu, F.; Yang, M.; Wang, Z.; Shi, Y.; Nan, B.; Yu, S.; Sun, Z.; Liu, Y.; Lu, Z. Highly durable organic electrode for sodium-ion batteries via a stabilized α-C radical intermediate. Nat. Commun. 2016, 7, 13318.
- 72 Fang, S.; Huang, J.; Tao, R.; Wei, Q.; Ding, X.; Yajima, S.; Chen, Z.; Zhu, W.; Liu, C.; Li, Y.; Yin, N.; Song, L.; Liu, Y.; Shi, G.; Wu, H.; Gao, Y.; Wen, X.; Chen, Q.; Shen, Q.; Li, Y.; Liu, Z.; Li, Y.; Ma, W. Open-Shell Diradical-Sensitized Electron Transport Layer for High-Performance Colloidal Quantum Dot Solar Cells. Adv. Mater. 2023, 35, 2212184.
- 73 Li, Y.; Li, L.; Wu, Y.; Li, Y. A Review on the Origin of Synthetic Metal Radical: Singlet Open-Shell Radical Ground State? J. Phys. Chem. C 2017, 121, 8579–8588.
- 74 Zhu, W.; Chen, Z.; Huang, J.; Liang, W.; Liao, C.; Wang, J.; Du, T.; Deng, Y.; Li, G.; Chen, R.; Peng, X.; Hou, J.; Li, Y. Open-Shell Donors and Closed-Shell Acceptors in Organic Solar Cells. J. Phys. Chem. C 2023, 127, 8894–8903.
- 75 Wang, J.; Lakraychi, A. E.; Liu, X.; Sieuw, L.; Morari, C.; Poizot, P.; Vlad, A. Conjugated sulfonamides as a class of organic lithium-ion positive electrodes. Nat. Mater. 2021, 20, 665–673.
- 76 Dong, H.; Tutusaus, O.; Liang, Y.; Zhang, Y.; Lebens-Higgins, Z.; Yang, W.; Mohtadi, R.; Yao, Y. High-power Mg batteries enabled by heterogeneous enolization redox chemistry and weakly coordinating electrolytes. Nat. Energy 2020, 5, 1043–1050.
- 77 Li, Y.; Lu, Y.; Ni, Y.; Zheng, S.; Yan, Z.; Zhang, K.; Zhao, Q.; Chen, J. Quinone Electrodes for Alkali–Acid Hybrid Batteries. J. Am. Chem. Soc. 2022, 144, 8066–8072.
- 78 Liang, Y.; Jing, Y.; Gheytani, S.; Lee, K.-Y.; Liu, P.; Facchetti, A.; Yao, Y. Universal quinone electrodes for long cycle life aqueous rechargeable batteries. Nat. Mater. 2017, 16, 841–848.
- 79 Dong, X.; Guo, Z.; Guo, Z.; Wang, Y.; Xia, Y. Organic Batteries Operated at −70°C. Joule 2018, 2, 902–913.
- 80 Hatakeyama-Sato, K.; Oyaizu, K. Redox: Organic Robust Radicals and Their Polymers for Energy Conversion/Storage Devices. Chem. Rev. 2023, 123, 11336–11391.
- 81 Joo, Y.; Agarkar, V.; Sung, S. H.; Savoie, B. M.; Boudouris, B. W. A nonconjugated radical polymer glass with high electrical conductivity. Science 2018, 359, 1391–1395.
- 82 Mi, Z.; Yang, P.; Wang, R.; Unruangsri, J.; Yang, W.; Wang, C.; Guo, J. Stable Radical Cation-Containing Covalent Organic Frameworks Exhibiting Remarkable Structure-Enhanced Photothermal Conversion. J. Am. Chem. Soc. 2019, 141, 14433–14442.
- 83 Gao, Y.; Liu, Y.; Li, X.; Wang, H.; Yang, Y.; Luo, Y.; Wan, Y.; Lee, C.-s.; Li, S.; Zhang, X.-H. A Stable Open-Shell Conjugated Diradical Polymer with Ultra-High Photothermal Conversion Efficiency for NIR-II Photo- Immunotherapy of Metastatic Tumor. Nano-Micro Lett. 2024, 16, 21.




