Free-Standing Multiscale Porous High Entropy NiFeCoZn Alloy as the Highly Active Bifunctional Electrocatalyst for Alkaline Water Splitting
Qiuping Zhang
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorXu Wang
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorTianzhen Jian
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorWenqing Ma
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorCorresponding Author
Caixia Xu
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
E-mail: [email protected][email protected]Search for more papers by this authorCorresponding Author
Qiuxia Zhou
School of Medical Information and Engineering, Southwest Medical University, Luzhou, Sichuan, 646000 China
E-mail: [email protected][email protected]Search for more papers by this authorHong Liu
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
State Key Laboratory of Crystal Materials, Shandong University, Jinan, Shandong, 250100 China
Search for more papers by this authorQiuping Zhang
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorXu Wang
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorTianzhen Jian
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorWenqing Ma
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
Search for more papers by this authorCorresponding Author
Caixia Xu
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
E-mail: [email protected][email protected]Search for more papers by this authorCorresponding Author
Qiuxia Zhou
School of Medical Information and Engineering, Southwest Medical University, Luzhou, Sichuan, 646000 China
E-mail: [email protected][email protected]Search for more papers by this authorHong Liu
Institute for Advanced Interdisciplinary Research (iAIR), Collaborative Innovation Center of Technology and Equipment for Biological Diagnosis and Therapy in Universities of Shandong, School of Chemistry and Chemical Engineering, University of Jinan, Jinan, Shandong, 250022 China
State Key Laboratory of Crystal Materials, Shandong University, Jinan, Shandong, 250100 China
Search for more papers by this authorComprehensive Summary
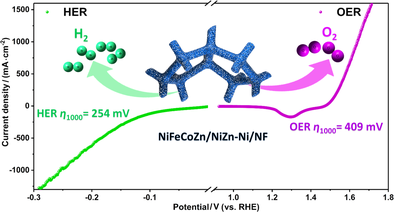
In the endeavor of searching for highly active and stable electrocatalysts toward overall water splitting, high-entropy-alloys have been the intense subjects owing to their advanced physicochemical property. The non-noble metal free-standing multiscale porous NiFeCoZn high-entropy-alloy is in situ constructed on the surface layer of NiZn intermetallic and Ni heterojunction over nickel foam (NiFeCoZn/NiZn-Ni/NF) by one scalable dealloying protocal to fulfill the outstanding bifunctional electrocatalytic performances toward overall water splitting. Because of the high-entropy effects and specific hierarchical porous architecture, the as-made NiFeCoZn/NiZn-Ni/ NF displays high intrinsic catalytic activities and durability toward both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in alkaline media. In particular, the in-situ construction of bimodal porous NiFeCoZn high-entropy-alloy results in the small overpotentials (η1000 = 254/409 mV for HER and OER), low Tafel slopes, and exceptional long-term catalytic durability for 400 h. Expressively, the electrolyzer constructed with NiFeCoZn/NiZn-Ni/NF as both cathode and anode exhibits a low cell voltage of 1.72 V to deliver the current density of 500 mA·cm–2 for overall water splitting. This work not only provides a facile and scalable protocol for the preparation of self-supporting high-entropy-alloy nanocatalysts but also enlightens the engineering of high performance bifunctional electrocatalysts toward water splitting.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202300718_sm_suppl.pdfPDF document, 1.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Li, Y. X.; Yu, X.; Gao, J.; Ma, Y. R. Structural and Electronic Modulation of (Fe,Ni)2P@Ni2P Heterostructure for Efficient Overall Water Splitting at High Current Density. Chem. Eng. J. 2023, 470, 144373.
- 2 Zhou, Y. B.; Shen, X. W.; Qian, T.; Yan, C. L.; Lu, J. M. A Review on the Rational Design and Fabrication of Nanosized High-Entropy Materials. Nano Res. 2023, 16, 7874–7905.
- 3 Feng, S. Y.; Rao, P.; Wu, X.; Li, K.; Qi, A. Y.; Yu, Y. H.; Li, J.; Deng, P. L.; Yuan, Y. L.; Wang, S. L.; Tian, X. L.; Kang, Z. Y. In-situ Generation of Hydroxyl Layers in CoO@FeSe2 Catalyst for High Selectivity Seawater Electrolysis. Chin. J. Chem. 2023, 1, 48–54.
- 4 Zhou, Q. X.; Hao, Q.; Li, Y. X.; Yu, J. H.; Xu, C. X.; Liu, H.; Yan, S. S. Free-Standing Trimodal Porous NiZn Intermetallic and Ni Heterojunction as Highly Efficient Hydrogen Evolution Electrocatalyst in the Alkaline Electrolyte. Nano Energy 2021, 89, 106402.
- 5 Zhou, J.; Huang, P.; Hao, Q.; Zhang, L. N.; Liu, H.; Xu, C. X.; Yu, J. H. Ag Nanoparticles Anchored on Nanoporous Ge Skeleton as High-Performance Anode for Lithium-ion Batteries. Chin. J. Chem. 2021, 39, 2881–2888.
- 6 Feng, D. Y.; Dong, Y. B.; Nie, P.; Zhang, L.; Qiao, Z. An CoNiCuMgZn High Entropy Alloy Nanoparticles Embedded onto Graphene Sheets via Anchoring and Alloying Strategy as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Chem. Eng. J. 2022, 430, 132883.
- 7 Shi, H.; Sun, X. Y.; Zeng, S. P.; Liu, Y.; Han, G. F.; Wang, T. H.; Wen, Z.; Fang, Q. R.; Lang, X. Y.; Jiang, Q. Nanoporous Nonprecious High-Entropy Alloys as Multisite Electrocatalysts for Ampere-Level Current- Density Hydrogen Evolution. Small Struct. 2023, 4, 2300042.
- 8 Zhou, P. F.; Liu, D.; Chen, Y. Y.; Chen, M. P.; Liu, Y. X.; Chen, S.; Kwok, C. T.; Tang, Y. X.; Wang, S. P.; Pan, H. Corrosion Engineering Boosting Bulk Fe50Mn30Co10Cr10 High-Entropy Alloy as High-Efficient Alkaline Oxygen Evolution Reaction Electrocatalyst. J. Mater. Sci. Technol. 2022, 109, 267–275.
- 9 Li, G. R.; Yin, F. X.; Lei, Z. P.; Zhao, X. R.; He, X. B.; Li, Z. C.; Yu, X. T. Se-doped Cobalt Oxide Nanoparticle as Highly-Efficient Electrocatalyst for Oxygen Evolution Reaction. Int. J. Hydrogen Energy 2022, 47, 216–227.
- 10 Zhou, Q. X.; Xu, C. X.; Li, Y. X.; Xie, X. M.; Liu, H.; Yan, S. S. Synergistic Coupling of NiFeZn-OH Nanosheet Network Arrays on a Hierarchical Porous NiZn/Ni Heterostructure for Highly Efficient Water Splitting. Sci. China Mater. 2021, 65, 1207–1216.
- 11 Zhang, B. L.; Xu, H.; Chen, Q.; Chen, H. Q.; He, G. Y. ZIF-67 Derived Mo2N/Mo2C Heterostructure as High-Efficiency Electrocatalyst for Hydrogen Evolution Reaction. J. Alloys Compd. 2022, 922, 166216.
- 12 Xiao, Y. L.; Yang, H.; Gong, X.; Hu, L.; Tong, Y. X.; Zhang, J. Y. Electrochemical Activation of Heterometallic Nanofibers for Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 2393–2401.
- 13 Wu, Z. C.; Zou, Z. X.; Huang, J. S.; Gao, F. NiFe2O4 Nanoparticles/NiFe Layered Double-Hydroxide Nanosheet Heterostructure Array for Efficient Overall Water Splitting at Large Current Densities. ACS Appl. Mater. Interfaces 2018, 10, 26283–26292.
- 14 Zhang, G. L.; Ming, K. S.; Kang, J. L.; Huang, Q.; Zhang, Z. J.; Zheng, X. R.; Bi, X. F. High Entropy Alloy as a Highly Active and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2018, 279, 19–23.
- 15 Jia, Z.; Yang, T.; Sun, L. G.; Zhao, Y. L.; Li, W. P.; Luan, J. H.; Lyu, F. C.; Zhang, L. C.; Kruzic, J. J.; Kai, J. J.; Huang, J. C.; Lu, J.; Liu, C. T. A Novel Multinary Nntermetallic as an Active Electrocatalyst for Hydrogen Evolution. Adv. Mater. 2020, 32, 2000385.
- 16 Lei, Y. T.; Zhang, L. L.; Xu, W. J.; Xiong, C. L.; Chen, W. X.; Xiang, X.; Zhang, B.; Shang, H. S. Carbon-Supported High-Entropy Co-Zn-Cd-Cu- Mn Sulfide Nanoarrays Promise High-Performance Overall Water Splitting. Nano Res. 2022, 15, 6054–6061.
- 17 Gao, S. J.; Hao, S. Y.; Huang, Z. N.;Yuan, Y. F.; Han, S.; Lei, L. C.; Zhang, X. W.; Shahbazian-Yassar, R.; Lu, J. Synthesis of High-Entropy Alloy Nanoparticles on Supports by the Fast Moving Bed Pyrolysis. Nat. Commun. 2020, 11, 2016.
- 18 Liu, C.; Zhu, H.; Lu, S. L.; Duan, F.; Du, M. L. High Entropy Alloy Nitrides with Integrated Nanowire/Nanosheet Architecture for Efficient Alkaline Hydrogen Evolution Reactions. New J. Chem. 2021, 45, 22255–22260.
- 19 Huang, K.; Xia, J. Y.; Lu, Y.; Zhang, B. W.; Shi, W. C.; Cao, X.; Zhang, X. Y.; Woods, L. M.; Han, C. C.; Chen, C. J.; Wang, T.; Wu, J. S.; Huang, Y. Z. Self-Reconstructed Spinel Surface Structure Enabling the Long- Term Stable Hydrogen Evolution Reaction/Oxygen Evolution Reaction Efficiency of FeCoNiRu High-Entropy Alloyed Electrocatalyst. Adv. Sci. 2023, 10, 2300094.
- 20 McKay, F.; Fang, Y. X.; Kizilkaya, O.; Singh, P.; Johnson, D. D.; Roy, A.; Young, D. P.; Sprunger, P. T.; Flake, J. C.; Shelton, W. A.; Xu, Y. CoCrFeNi High-Entropy Alloy as an Enhanced Hydrogen Evolution Catalyst in an Acidic Solution. J. Phys. Chem. C 2021, 125, 17008–17018.
- 21 Zhang, D.; Shi, Y.; Zhao, H.; Qi, W. J.; Chen, X. L.; Zhan, T. R.; Li, S. X.; Yang, B.; Sun, M. Z.; Lai, J. P.; Huang, B. L.; Wang, L. The Facile Oil-Phase Synthesis of a Multi-Site Synergistic High-Entropy Alloy to Promote the Alkaline Hydrogen Evolution Reaction. J. Mater. Chem. A 2021, 9, 889–893.
- 22 Waag, F.; Li, Y.; Ziefuß, A. R.; Bertin, E.; Kamp, M.; Duppel, V.; Marzun, G.; Kienle, L.; Barcikowski, S.; Gökce, B. Kinetically-Controlled Laser-Synthesis of Colloidal High-Entropy Alloy Nanoparticles. RSC Adv. 2019, 9, 18547–18558.
- 23 Yao, Y. G.; Huang, Z. N.; Xie, P. F.; Lacey, S. D.; Jacob, R. J.; Xie, H.; Chen, F. J.; Nie, A.; Pu, T. C.; Rehwoldt, M.; Yu, D. W.; Zachariah, M. R.; Wang, C.; Shahbazian-Yassar, R.; Li, J.; Hu, L. B. Carbothermal Shock Synthesis of High-Entropy-Alloy Nanoparticles. Nature 2018, 359, 1489–1494.
- 24 Bondesgaard, M.; Broge, N. N.; Mamakhel, A.; Bremholm, M.; Iversen, B. B. General Solvothermal Synthesis Method for Complete Solubility Range Bimetallic and High-Entropy Alloy Nanocatalysts. Adv. Funct. Mater. 2019, 29, 1905933.
- 25 Li, K.; Chen, W. Recent Progress in High-Entropy Alloys for Catalysts: Synthesis, Applications, and Prospects. Mater. Today Energy 2021, 20, 100638.
- 26 Wang, S. Q.; Xu, B. L.; Huo, W. Y.; Feng, H. C.; Zhou, X. F.; Fang, F.; Xie, Z. H.; Shang, J. K.; Jiang, J. Q. Efficient FeCoNiCuPd Thin-Film Electrocatalyst for Alkaline Oxygen and Hydrogen Evolution Reactions. Appl. Catal. B Environ. 2022, 313, 121472.
- 27 Shi, H.; Sun, X. Y.; Liu, Y.; Zeng, S. P.; Zhang, Q. H.; Gu, L.; Wang, T. H.; Han, G. F.; Wen, Z.; Fang, Q. R.; Lang, X. Y.; Jiang, Q. Multicomponent Intermetallic Nanoparticles on Hierarchical Metal Network as Versatile Electrocatalysts for Highly Efficient Water Splitting. Adv. Funct. Mater. 2023, 33, 2214412.
- 28 Yao, R. Q.; Zhou, Y. T.; Shi, H.; Wan, W. B.; Zhang, Q. H.; Gu, L.; Zhu, Y. F.; Wen, Z.; Lang, X. Y; Jiang, Q. Nanoporous Surface High-Entropy Alloys as Highly Efficient Multisite Electrocatalysts for Nonacidic Hydrogen Evolution Reaction. Adv. Funct. Mater. 2020, 31, 2300042.
- 29 Peng, H. L.; Xie, Y. C. Z.; Xie, Z. C.; Wu, Y. F.; Zhu, W. K.; Liang, S. Q.; Wang, L. B. Large-scale and Facile Synthesis of a Porous High-Entropy Alloy CrMnFeCoNi as an Efficient Catalyst. J. Mater. Chem. A 2020, 8, 18318–18326.
- 30
Yu, T. T.; Zhang, Y. Y.; Hu, Y. X.; Hu, K. L.; Lin, X.; Xie, G. Q.; Liu, X. J.; Reddy, K. M.; Ito, Y.; Qiu, H. J. Twelve-Component Free-Standing Nanoporous High-Entropy Alloys for Multifunctional Electrocatalysis. ACS Mater. Lett. 2021, 4, 181–189.
10.1021/acsmaterialslett.1c00762 Google Scholar
- 31 Zhang, Y.; Zhou, Y. J.; Lin, J. P.; Chen, G. L.; Liaw, P. K. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538.
- 32 Zhou, Q. X.; Xu, C. X.; Hou, J. G.; Ma, W. Q.; Jian, T. Z.; Yan, S. S.; Liu, H. Duplex Interpenetrating-Phase FeNiZn and FeNi3 Heterostructure With Low-Gibbs Free Energy Interface Coupling for Highly Efficient Overall Water Splitting. Nano-Micro Lett. 2023, 15, 95.
- 33 Cai, Z. Y.; Bu, X. M.; Wang, P.; Su, W. Q.; Wei, R. J.; Ho, J. C.; Yang, J. H.; Wang, X. Y. Simple and Cost Effective Fabrication of 3D Porous Core–Shell Ni Nanochains@NiFe Layered Double Hydroxide Nanosheet Bifunctional Electrocatalysts for Overall Water Splitting. J. Mater. Chem. A 2019, 7, 21722–21729.
- 34 Mei, Y. J.; Feng, Y. B.; Zhang, C. X.; Zhang, Y.; Qi, Q. L.; Hu, J. High-Entropy Alloy with Mo-Coordination as Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Catal. 2022, 12, 10808–108178.
- 35 Liu, H. M.; Luo, Q. X.; Hu, J. P.; Wei, L. W.; Zhang, W. Q.; Zheng, H.; Wu, S. S.; Tang, K. B. Iridium-Doped 10H-Phase Perovskite BaCo0.8Fe0.15Ir0.05O3–δ as an Efficient Oxygen Evolution Reaction Catalyst. Chin. J. Chem. 2022, 40, 2276–2284.
- 36 Das, M.; Kumar, G.; Dey, R. Sundar Electrochemical Growth and Formation Mechanism of Cu2Se/CoSe2-Based Bifunctional Electrocatalyst: A Strategy for the Development of Efficient Material Toward Water Electrolysis. ACS Appl. Energy Mater. 2022, 5, 3915–3925.
- 37 Jin, Z. Y.; Lv, J.; Jia, H. L.; Liu, W. H.; Li, H. L.; Chen, Z. H.; Lin, X.; Xie, G. Q.; Liu, X. J.; Sun, S. H.; Qiu, H. J. Nanoporous Al-Ni-Co-Ir-Mo High-Entropy Alloy for Record-High Water Splitting Activity in Acidic Environments. Small 2019, 15, 1904180.
- 38 Ding, Z. Y.; Bian, J. J.; Shuang, S.; Liu, X. D.; Hu, Y. C.; Sun, C. W.; Yang, Y. High Entropy Intermetallic-Oxide Core-Shell Nanostructure as Superb Oxygen Evolution Reaction Catalyst. ACS Appl. Energy Mater. 2020, 4, 1900105.
- 39 Ding, K. X.; Hu, J. G.; Zhao, L. M.; Yu, H. N.; Cai, S.; Yang, Y.; Tan, J.; Hou, H. S.; Ji, X. B. Dual-confined bead-like CoSe2@NC@NCNFs bifunctional catalyst boosting rechargeable zinc-air batteries. Chin. J. Chem. 2023, 4, 397–405.
- 40 Nguyen, T. X.; Su, Y. H.; Lin, C. C.; Ruan, J.; Ting, J. M. A New High Entropy Glycerate for High Performance Oxygen Evolution Reaction. Adv. Sci. 2021, 8, 2002446.
- 41 Sharma, L.; Katiyar, N. K.; Parui, A.; Das, R.; Kumar, R.; Tiwary, C. S.; Singh, A. K.; Halder, A.; Biswas, K. Low-Cost High Entropy Alloy (HEA) for High-Efficiency Oxygen Evolution Reaction (OER). Nano Res. 2021, 15, 4799–4806.
- 42 Wang, X. X.; She, G. W.; Mu, L. X.; Shi, W. S. Amorphous Co–Mo–P–O Bifunctional Electrocatalyst via Facile Electrodeposition for Overall Water Splitting. ACS Sustainable Chem. Eng. 2020, 8, 2835–2842.
- 43 Li, Z. J.; Jang, H.; Qin, D.; Jiang, X. L.; Ji, X. Q.; Kim, M. G.; Zhang, L. J.; Liu, X.; Cho, J. Alloy-Strain-Output Induced Lattice Dislocation in Ni3FeN/Ni3Fe Ultrathin Nanosheets for Highly Efficient Overall Water Splitting. J. Mater. Chem. A 2021, 9, 4036–4043.
- 44 Zhang, Q.; Zhang, R. X.; Zhao, Y. X.; Sun, T. T.; Gao, J. Y.; Xu, G. R.; Wu, Z. X.; Yang, Y.; Wang, L. Surface/Interface Engineering of Hierarchical MoO2/MoNi4@Ru/RuO2 Heterogeneous Nanosheet Arrays for Alkaline Water Electrolysis with Fast Hinetics. Chin. J. Chem. 2023, 4, 119–128.
- 45 Xu, Z. C.; Yeh, C. L.; Chen, J. L.; Lin, J. T.; Ho, K. C.; Lin, R. Y. Y. Metal-Organic Framework-Derived 2D NiCoP Nanoflakes From Layered Double Hydroxide Nanosheets for Efficient Electrocatalytic Water Splitting at High Current Densities. ACS Sustainable Chem. Eng. 2022, 10, 11577–11586.
- 46 He, R. Z.; Wang, C. Y.; Feng, L. G. Amorphous FeCoNi-S as Efficient Bifunctional Electrocatalysts for Overall Water Splitting Reaction. Chin. Chem. Lett. 2023, 34, 107241.
- 47 Chen, J.; Pan, A. Q.; Zhang, W. C.; Cao, X. X.; Lu, R.; Liang, S. Q.; Cao, G. Z. Melamine-Assisted Synthesis of Ultrafine Mo2C/Mo2N@N- doped Carbon Nanofibers for Enhanced Alkaline Hydrogen Evolution Reaction Activity. Sci. China. Mater. 2020, 64, 1150–1158.
- 48 Wu, Z. C.; Zou, Z. X.; Huang, J. S.; Gao, F. Fe-doped NiO mesoporous nanosheets array for highly efficient overall water splitting. J. Catal. 2018, 358, 243–252.
- 49 Chen, W. Q.; Yan, X.; Liu, Z. L.; Zhang, X. C.; Du, C. F. Flower-like HEA/MoS2/MoP Heterostructure Based on Interface Engineering for Efficient Overall Water Splitting. Int. J. Hydrogen Energy 2023, 48, 29969–29981.
- 50 Qin, H. C.; He, Z. Y.; Hu, K. L.; Hu, P. Y.; Dai, R.; Wang, Z. P. CuO Nanosheets Prepared by Dielectric Barrier Discharge Microplasma as Catalysts for the Oxygen Evolution Reaction. ACS Appl. Nano Mater. 2022, 5, 14689–14696.
- 51 Zhang, Z.; Li, X. P.; Zhong, C.; Zhao, N. Q.; Deng, Y. D.; Han, X. P.; Hu, W. B. Spontaneous Synthesis of Silver-Nanoparticle-Decorated Transition-Metal Hydroxides for Enhanced Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2020, 59, 7245–7250.
- 52 Yu, M. Z.; Zheng, J. Q.; Guo, M. La-Doped NiFe-LDH Coupled with Hierarchical Vertically Aligned MXene Frameworks for Efficient Overall Water Splitting. J. Energy Chem. 2022, 70, 472–479.
- 53 Hu, H. S.; Li, Y.; Shao, Y. R.; Li, K. X.; Deng, G.; Wang, C. B.; Feng, Y. Y. NiCoP Nanorod Arrays as High-Performance Bifunctional Electrocatalyst for Overall Water Splitting at High Current Densities. J. Power Sources 2021, 484, 229269.
- 54 Yao, M. Q.; Hu, H. H.; Sun, B. L.; Wang, N.; Hu, W. C.; Komarneni, S. Self-Supportive Mesoporous Ni/Co/Fe Phosphosulfide Nanorods Derived from Novel Nydrothermal Electrodeposition as a Highly Efficient Electrocatalyst for Overall Water Splitting. Small 2019, 15, 1905201.
- 55 Liu, H.; Xi, C.; Xin, J. H,; Zhang, G. L.; Zhang, S. F.; Zhang, Z. J.; Huang, Q.; Li, J. X.; Liu, H.; Kang, J. L. Free-Standing Nanoporous NiMnFeMo Alloy: An Efficient Non-Precious Metal Electrocatalyst for Water Splitting. Chem. Eng. J. 2021, 404, 126530.
- 56 Wang, X. M.; Ma, W. G.; Ding, C. M.; Xu, Z. Q.; Wang, H.; Zong, X.; Li, C. Amorphous Multi-elements Electrocatalysts with Tunable Bifunctionality toward Overall Water Splitting. ACS Catal. 2018, 8, 9926–9935.
- 57 Tian, G. Q.; Wei, S. R.; Guo, Z. T.; Wu, S. W.; Chen, Z. L.; Xu, F. M.; Cao, Y.; Liu, Z.; Wang, J. Q.; Ding, L.; Tu, J. C.; Zeng, H. Hierarchical NiMoP2-Ni2P with Amorphous Interface as Superior Bifunctional Electrocatalysts for Overall Water Splitting. J. Mater. Sci. Technol. 2021, 77, 108–116.




