Synergistic Brønsted Base/Photoredox-Catalyzed Three-Component Coupling with Malonates to Synthesize δ-Hydroxy Esters and δ-Keto Esters
Ting Li
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
These authors contributed equally.
Search for more papers by this authorWei Wang
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
These authors contributed equally.
Search for more papers by this authorMing Dong
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorZhijie Zhang
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorSha Yu
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorZhengchu Chen
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorCorresponding Author
Siping Wei
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
*E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dong Yi
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Luzhou, Sichuan, 646000 China
*E-mail: [email protected]; [email protected]Search for more papers by this authorTing Li
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
These authors contributed equally.
Search for more papers by this authorWei Wang
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
These authors contributed equally.
Search for more papers by this authorMing Dong
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorZhijie Zhang
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorSha Yu
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorZhengchu Chen
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Search for more papers by this authorCorresponding Author
Siping Wei
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
*E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Dong Yi
Green Pharmaceutical Technology Key Laboratory of Luzhou City, Central Nervous System Drug Key Laboratory of Sichuan Province, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, 646000 China
Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Luzhou, Sichuan, 646000 China
*E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Multicomponent alkene 1,2-dicarbofunctionalizations (DCFs) have emerged as a powerful strategy to rapidly incorporate both two carbon subunits across one C—C double bond in one step for enhancing molecular complexity and diversity. To the best of our knowledge, there is only one report on photoredox-catalyzed three-component DCFs with malonates through the radical−radical cross-coupling, while photoredox-catalyzed radical-polar crossover (RPC)-type DCFs with malonates were still rare. Herein, we describe a redox-neutral RPC-type 1,2-dialkylation of styrenes with malonates and aldehydes through the synergistic Brønsted base/photoredox catalysis system. This transition-metal-free strategy provides an efficient and clean approach to a broad variety of δ-hydroxy esters and also features exceptionally mild conditions, wide compatibility of substrate scope and functional groups, and high atomic economy. Moreover, three-component 1,2-alkylacylation from the same starting materials was achieved in one-pot manner through such RPC-type coupling and subsequent two-electron oxidation process, providing a set of δ-keto esters of interest in pharmaceutical research.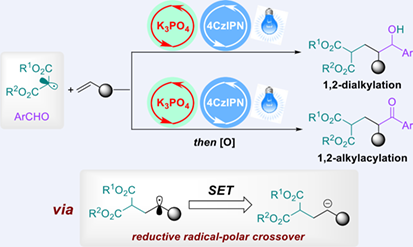
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300683-sup-0001-supinfo.pdfPDF document, 8.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673; (b) Van Lommel, R.; Bock, J.; Daniliuc, C. G.; Hennecke, U.; De Proft, F. A Dynamic Picture of the Halolactonization Reaction through a Combination of ab initio Metadynamics and Experimental Investigations. Chem. Sci. 2021, 12, 7746–7757; (c) White, D. R.; Hinds, E. M.; Bornowski, E. C.; Wolfe, J. P. Pd-Catalyzed Alkene Difunctionalization Reactions of Malonate Nucleophiles: Synthesis of Substituted Cyclopentanes via Alkene Aryl-Alkylation and Akenyl-Alkylation. Org. Lett. 2019, 21, 3813–3816.
- 2(a) Dénès, F.; Pérez-Luna, A.; Chemla, F. Addition of Metal Enolate Derivatives to Unactivated Carbon−Carbon Multiple Bonds. Chem. Rev. 2010, 110, 2366–2447; (b) Dong, Z.; Ren, Z.; Thompson, S. J.; Xu, Y.; Dong, G. Transition-Metal-Catalyzed C–H Alkylation Using Alkenes. Chem. Rev. 2017, 117, 9333–9403.
- 3(a) Gu, G.; Huang, M.; Kim, J. K.; Zhang, J.; Li, Y.; Wu, Y. Visible- Light-Induced Photocatalyst-Free C-3 Functionalization of Indoles with Diethyl Bromomalonate. Green Chem. 2020, 22, 2543–2548; (b) Hirase, K.; Iwahama, T.; Sakaguchi, S.; Ishii, Y. Catalytic Radical Addition of Carbonyl Compounds to Alkenes by Mn(II)/Co(II)/O2 System. J. Org. Chem. 2002, 67, 970–973; (c) Ko, T. Y.; Youn, S. W. Cooperative Indium(III)/Silver(I) System for Oxidative Coupling/Annulation of 1,3-Dicarbonyls and Styrenes: Construction of Five-Membered Heterocycles. Adv. Synth. Catal. 2016, 358, 1934–1941.
- 4(a) Campbell, M. W.; Compton, J. S.; Kelly, C. B.; Molander, G. A. Three-Component Olefin Dicarbofunctionalization Enabled by Nickel/ Photoredox Dual Catalysis. J. Am. Chem. Soc. 2019, 141, 20069–20078; (b) Fang, H.; Empel, C.; Atodiresei, I.; Koenigs, R. M. Photoinduced Palladium-Catalyzed 1,2-Difunctionalization of Electron-Rich Olefins via a Reductive Radical-Polar Crossover Reaction. ACS Catal. 2023, 13, 6445–6451; (c) Liu, Y.; Liu, H.; Liu, X.; Chen, Z. Recent Advances in Photoredox-Catalyzed Difunctionalization of Alkenes. Catalysts 2023, 13, 1056; (d) Patel, M.; Desai, B.; Sheth, A.; Dholakiya, B. Z.; Naveen, T. Recent Advances in Mono- and Difunctionalization of Unactivated Olefins. Asian J. Org. Chem. 2021, 10, 3201–3232; (e) Zhang, M.; Zhang, J.; Zhou, Y.; He, Y.; Wu, J.; Zheng, D. Photoredox- Catalyzed Three-Component Amidoheteroarylation of Unactivated Alkenes. Org. Lett. 2023, 25, 4113–4118; (f) Pan, C.; Meng, Y.; Deng, Y.; Zhou, B.; Chen, J.; He, Z.; Sun, W.; Khan, R.; Fan, B. Metal-Free Visible-Light-Induced Atom-Transfer Radical Addition Reaction of Alkenes/Alkynes with ICH2CN. Chin. J. Chem. 2022, 40, 2040–2046; (g) Wang, L.; Zhang, H.; Zhu, C.; Feng, C. Expedient Trifluoromethylacylation of Styrenes Enabled by Photoredox Catalysis. Chin. J. Chem. 2022, 40, 59–64; (h) Yang, M.; Wang, X. Y.; Wang, J.; Zhao, Y. L. Visible Light-Induced [3+2] Annulation Reaction of Alkenes with Vinyl Azides: Direct Synthesis of Functionalized Pyrroles. Chin. J. Chem. 2024, 42, 151–156; (i) Zhang, F.; Liao, S.; Zhou, L.; Yang, K.; Wang, C.; Lou, Y.; Wang, C.; Song, Q. An Olefinic 1,2-α-Boryl Migration Enables 1,2-Bis(boronic esters) via Radical-Polar Crossover Reaction. Chin. J. Chem. 2022, 40, 582–588; (j) Zhang, M.; Li, Q.; Lin, J. H.; Xiao, J. C. Difluoroalkylation/Lactonization of Alkenes with BrCF2CO2K via Photoredox Catalysis: Access to α,α-Difluoro-γ-lactones. Chin. J. Chem. 2023, 41, 2819–2824; (k) Sun, Q.; Zhang, X.-P.; Duan, X.; Qin, L.-Z.; Yuan, X.; Wu, M.-Y.; Liu, J.; Zhu, S.-S.; Qiu, J.-K.; Guo, K. Photoinduced Merging with Copper- or Nickel-Catalyzed 1,4-Cyanoalkylarylation of 1,3-Enynes to Access Multiple Functionalizatized Allenes in Batch and Continuous Flow. Chin. J. Chem. 2022, 40, 1537–1545; (l) Wang, Z.; Liu, Q.; Liu, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Visible-Light-Initiated 4CzIPN Catalyzed Multi-Component Tandem Reactions to Assemble Sulfonated Quinoxalin-2(1H)-Ones. Chin. Chem. Lett. 2022, 33, 1479–1482; (m) Zhang, Y.; Jiang, W.; Bao, X.; Qiu, Y.; Yuan, Y.; Yang, C.; Huo, C. Photocatalyzed Reverse Polarity Oxidative Povarov Reaction of Glycine Derivatives with Maleimides. Chin. J. Chem. 2021, 39, 3238–3244; (n) Wang, Z.; Meng, N.; Lv, Y.; Wei, W.; Yue, H.; Zhong, G. Photocatalyst-Free Visible-Light-Mediated Three-Component Reaction of α-Diazoesters, Cyclic Ethers and NaSCN to Access Organic Thiocyanates. Chin. Chem. Lett. 2023, 34, 107599; (o) Li, S.; Chen, Q.; Li, W.; Gu, G.; Zhang, J. Visible Light Driven Copper(I) Catalyzed Oxyamination of Electron Deficient Alkenes. Chin. J. Chem. 2020, 38, 1116–1122; (p) Su, Z.; Guo, Y.; Chen, Q.-Y.; Zhao, Z.-G.; Nian, B.-Y. Catalyst-Free Hydroxytrifluoromethylation of Alkenes UsingIodotrifluoromethane. Chin. J. Chem. 2019, 37, 597–604; (q) Yang, B.; Ren, X.; Shen, X.; Li, T.; Lu, Z. Visible Light-Promoted Three-Component Carboazidation of Unactivated Alkenes with TMSN3 and Acrylonitrile. Chin. J. Chem. 2018, 36, 1017–1023.
- 5(a) Katta, N.; Zhao, Q.-Q.; Mandal, T.; Reiser, O. Divergent and Synergistic Photocatalysis: Hydro- and Oxoalkylation of Vinyl Arenes for the Stereoselective Synthesis of Cyclopentanols via a Formal [4+1]-Annulation of 1,3-Dicarbonyls. ACS Catal. 2022, 12, 14398–14407; (b) Poudel, D. P.; Pokhrel, A.; Tak, R. K.; Shankar, M.; Giri, R. Photosensitized O2 Enables Intermolecular Alkene Cyclopropanation by Active Methylene Compounds. Science 2023, 381, 545–553.
- 6(a) Lei, G.; Xu, M.; Chang, R.; Funes-Ardoiz, I.; Ye, J. Hydroalkylation of Unactivated Olefins via Visible-Light-Driven Dual Hydrogen Atom Transfer Catalysis. J. Am. Chem. Soc. 2021, 143, 11251–11261; (b) Yamashita, Y.; Ogasawara, Y.; Banik, T.; Kobayashi, S. Photoinduced Efficient Catalytic α-Alkylation Reactions of Active Methylene and Methine Compounds with Nonactivated Alkenes. J. Am. Chem. Soc. 2023, 145, 23160–23166.
- 7 Hong, Y.; Dong, M.-Y.; Li, D.-S.; Deng, H.-P. Photoinduced Three- Component Carboarylation of Unactivated Alkenes with Protic C(sp3)–H Feedstocks. Org. Lett. 2022, 24, 7677–7684.
- 8 Baś, S.; Yamashita, Y.; Kobayashi, S. Development of Brønsted Base–Photocatalyst Hybrid Systems for Highly Efficient C–C Bond Formation Reactions of Malonates with Styrenes. ACS Catal. 2020, 10, 10546–10550.
- 9(a) Li, W.; Tan, L.; Chen, Y.; Liu, R.; Qi, Z.; Yuan, S.; Huang, Z.; Wei, S.; Du, X.; Yi, D. Metal-Free Photocatalytic [4+2] Annulation of Acrylamides with 2-Benzyl-2-bromocarbonyls to Assemble Tetralin-1-carboxamides. Chin. J. Chem. 2024, 42, 157–163; (b) Liu, R.; Zou, T.; Yu, S.; Li, W.; Wei, S.; Gong, Y.; Zhang, Z.; Zhang, S.; Yi, D. Photoredox- Catalyzed Three-Component 1,2-Cyanoalkylpyridylation of Styrenes with Nonredox-Active Cyclic Oximes. J. Org. Chem. 2023, 88, 16410–16423; (c) Qi, Z.; Zhang, Z.; Yang, L.; Zhang, D.; Lu, J.; Wei, J.; Wei, S.; Fu, Q.; Du, X.; Yi, D. Nitrogen-Radical-Triggered Trifunctionalizing Ipso-Spirocyclization of Unactivated Alkenes with Vinyl Azides: A Modular Access to Spiroaminal Frameworks. Adv. Synth. Catal. 2021, 363, 3762–3768; (d) Tu, S.; Qi, Z.; Li, W.; Zhang, S.; Zhang, Z.; Wei, J.; Yang, L.; Wei, S.; Du, X.; Yi, D. Chemodivergent Photocatalytic Access to 1-Pyrrolines and 1-Tetralones Involving Switchable C(sp3)–H Functionalization. Front. Chem. 2022, 10, 1058596; (e) Lv, Y.; Ding, H.; You, J.; Wei, W.; Yi, D. Additive-Free Synthesis of S-substituted Isothioureas via Visible-Light-Induced Four-Component Reaction of α-Diazoesters, Aryl Isothiocyanates, Amines and Cyclic Ethers. Chin. Chem. Lett. 2024, 35, 109107; (f) Yi, D.; He, L.; Qi, Z.; Zhang, Z.; Li, M.; Lu, J.; Wei, J.; Du, X.; Fu, Q.; Wei, S. Copper-Catalyzed Aerobic Oxidative Cleavage of Unstrained Carbon-Carbon Bonds of 1,1-Disubstituted Alkenes with Sulfonyl Hydrazides. Chin. J. Chem. 2021, 39, 859–865; (g) Zhang, M.; Zhang, Z.; He, Y.; Zou, T.; Qi, Z.; Fu, Q.; Wei, J.; Lu, J.; Wei, S.; Yi, D. Photocatalytic Deoxygenative Carboimination towards Functionalized Pyrrolines by Using Unstrained γ,δ-Unsaturated Oximes. Adv. Synth. Catal. 2021, 363, 2110–2116.
- 10(a) Hasdemir, B. Asymmetric Synthesis of Some Chiral Aryl and Hetero Aryl-Substituted β-, γ-, δ-Hydroxy Esters. Synth. Commun. 2015, 45, 1082–1088; (b) Mehl, F.; Bombarda, I.; Vanthuyne, N.; Faure, R.; Gaydou, E. M. Hemisynthesis and Odour Properties of δ-Hydroxy-γ-Lactones and Precursors Derived from Linalool. Food Chem. 2010, 121, 98–104.
- 11(a) Díaz-Rodríguez, A.; Borzęcka, W.; Lavandera, I.; Gotor, V. Stereodivergent Preparation of Valuable γ- or δ-Hydroxy Esters and Lactones through One-Pot Cascade or Tandem Chemoenzymatic Protocols. ACS Catal. 2014, 4, 386–393; (b) Kopp, N.; Civenni, G.; Marson, D.; Laurini, E.; Pricl, S.; Catapano, C. V.; Humpf, H.-U.; Almansa, C.; Nieto, F. R.; Schepmann, D.; Wünsch, B. Chemoenzymatic Synthesis of 2,6-Disubstituted Tetrahydropyrans with High σ1 Receptor Affinity, Antitumor and Analgesic Activity. Eur. J. Med. Chem. 2021, 219, 113443; (c) Pàmies, O.; Bäckvall, J.-E. Enzymatic Kinetic Resolution and Chemoenzymatic Dynamic Kinetic Resolution of δ-Hydroxy Esters. An Efficient Route to Chiral δ-Lactones. J. Org. Chem. 2002, 67, 1261–1265.
- 12 Speckmeier, E.; Fischer, T. G.; Zeitler, K. A Toolbox Approach to Construct Broadly Applicable Metal-Free Catalysts for Photoredox Chemistry: Deliberate Tuning of Redox Potentials and Importance of Halogens in Donor–Acceptor Cyanoarenes. J. Am. Chem. Soc. 2018, 140, 15353–15365.
- 13(a) Bao, Q.-F.; Li, M.; Xia, Y.; Wang, Y.-Z.; Zhou, Z.-Z.; Liang, Y.-M. Visible-Light-Mediated Decarboxylative Radical Addition Bifunctionalization Cascade for the Production of 1,4-Amino Alcohols. Org. Lett. 2021, 23, 1107–1112; (b) Bao, Q.-F.; Xia, Y.; Li, M.; Wang, Y.-Z.; Liang, Y.-M. Visible-Light-Mediated Trifluoromethylation/Benzylation of Styrenes Catalyzed by 4-CzIPN. Org. Lett. 2020, 22, 7757–7761; (c) Sun, W.-H.; Zou, J.-Y.; Xu, X.-J.; Wang, J.-L.; Liu, M.-L.; Liu, X.-Y. Photo-Catalyzed Redox-Neutral 1,2-Dialkylation of Alkenes. Adv. Synth. Catal. 2022, 364, 2260–2265; (d) Venditto, N. J.; Boerth, J. A. Photoredox-Catalyzed Multicomponent Synthesis of Functionalized γ-Amino Butyric Acids via Reductive Radical Polar Crossover. Org. Lett. 2023, 25, 3429–3434; (e) Wang, A.; Yin, Y.-Y.; Rukhsana; Wang, L.-Q.; Jin, J.-H.; Shen, Y.-M. Visible-Light-Mediated Three-Component Decarboxylative Coupling Reactions to Synthesize 1,4-Diol Monoethers. J. Org. Chem. 2023, 88, 13871–13882.
- 14 Sim, B. A.; Milne, P. H.; Griller, D.; Wayner, D. D. M. Thermodynamic Significance of ρ+ and ρ- from Substituent Effects on the Redox Potentials of Arylmethyl Radicals. J. Am. Chem. Soc. 1990, 112, 6635–6638.
- 15 Roth, H. G.; Romero, N. A.; Nicewicz, D. A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single-Electron Redox Chemistry. Synlett 2016, 27, 714–723.
- 16 Ota, K.; Nagao, K.; Ohmiya, H. Synthesis of Sterically Hindered α-Hydroxycarbonyls through Radical-Radical Coupling. Org. Lett. 2021, 23, 4420–4425.
- 17(a) Leifert, D.; Studer, A. The Persistent Radical Effect in Organic Synthesis. Angew. Chem. Int. Ed. 2020, 59, 74–108; (b) Song, Q.; Zhao, H.; Sun, Y.; Jiang, H.; Zhang, M. Direct C(sp3)–H Sulfonylation of Xanthene Derivatives with Sodium Sulfinates by Oxidative Copper Catalysis. Chin. J. Chem. 2022, 40, 371–377; (c) Wang, M.; Zhang, C.; Ci, C.; Jiang, H.; Dixneuf, P. H.; Zhang, M. Room Temperature Construction of Vicinal Amino Alcohols via Electroreductive Cross- Coupling of N-Heteroarenes and Carbonyls. J. Am. Chem. Soc. 2023, 145, 10967–10973; (d) Niu, T.; Liu, J.; Wu, X.; Zhu, C. Radical Heteroarylalkylation of Alkenes via Three-Component Docking- Migration Thioetherification Cascade. Chin. J. Chem. 2020, 38, 803–806.




