Nanomedicine Disrupts Stromal Barriers to Augment Drug Penetration for Improved Cancer Therapy†
Corresponding Author
Guo-Feng Luo
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Wuhan University, Wuhan, Hubei, 430079 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei-Hai Chen
Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xian-Zheng Zhang
Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guo-Feng Luo
State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, Key Laboratory of Oral Biomedicine Ministry of Education, Hubei Key Laboratory of Stomatology, School & Hospital of Stomatology, Wuhan University, Wuhan, Hubei, 430079 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wei-Hai Chen
Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xian-Zheng Zhang
Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorDedicated to the 130th Anniversary of Wuhan University.
Comprehensive Summary
Tumor stroma composing diverse extracellular matrixes (ECM) and
stromal cells shapes a condensed physical barrier, which severely hampers the efficient accessibility of nanomedicine to tumor cells, especially these deep-seated in the core of tumor. Such barrier significantly compromises the antitumor effects of drug-loaded nanomedicine, revealing the remarkable importance of disrupting stromal barrier for improved tumor therapy with deep penetration ability. To achieve this goal, various nanoparticle-based strategies have been developed, including direct depleting ECM components via delivering anti-fibrotic agents or targeting stromal cells to suppress ECM expression, dynamic regulation of nanoparticles’ physicochemical properties (i.e., size, surface charge, and morphology), mechanical force-driven deep penetration, natural/biomimetic self-driven nanomedicine, and transcytosis-inducing nanomedicine. All these nanostrategies were systemically summarized in this review, and the design principles for obtaining admirable nanomedicine were included. With the rapid development of nanotechnology, elaborate design of multifunctional nanomedicine provides new opportunities for overcoming the critical stromal barriers to maximize the therapeutic index of various therapies, such as chemotherapy, photodynamic therapy, and immunotherapy. 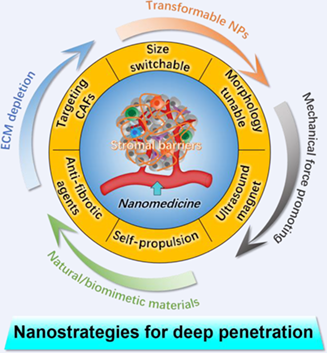
Key Scientists
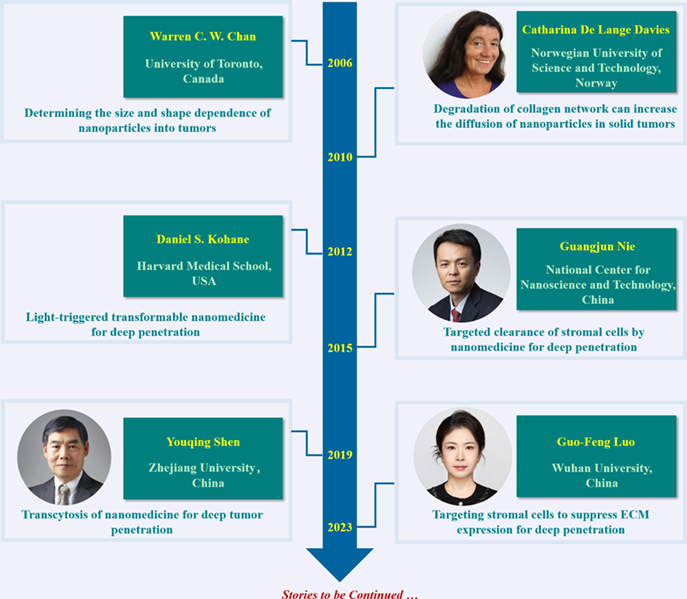
In 2006, Chan et al. demonstrated that the size and shape of nanoparticles were important for biomedical applications, such as intracellular delivery rate and tissue penetration. In addition, the degradation of the structural collagen was confirmed to increase the diffusion of nanoparticles and macromolecules by Davies et al. in 2010. These findings reveal the importance of chemophysical properties of nanoparticles in determining their diffusion and the critical roles of stromal barrier in hindering nanoparticles penetration. On the basis of this, photoswitchable nanoparticles were developed by Kohane et al. for triggered tissue penetration and efficient drug delivery. In 2015, Nie et al. reported the targeted depletion of cancer-associated fibroblasts by peptide assembly for enhanced antitumor drug delivery. In 2019, Shen et al. proposed the transcytosis strategy to promote the tumor penetration of nanoparticles. Very recently, Luo et al. reported the specific reversing of the biological function of cancer-associated fibroblasts to suppress the generation of stromal matrix, greatly increasing the drug perfusion in tumor tissue. All these strategies fueled the design and construction of function-specific nanoparticles for tumor therapy.
References
- 1 Shi, J.; Kantoff, P. W.; Wooster, R.; Farokhzad, O. C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2016, 17, 20–37.
- 2 van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W. J. M.; Lammers, T. Smart Cancer Nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017.
- 3 Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157.
- 4 Caruso, F.; Hyeon, T.; Rotello, V. M. Nanomedicine. Chem. Soc. Rev. 2012, 41, 2537–2538.
- 5 Huo, D.; Jiang, X.; Hu, Y. Recent Advances in Nanostrategies Capable of Overcoming Biological Barriers for Tumor Management. Adv. Mater. 2019, 32, e1904337.
- 6 Riley, R. S.; June, C. H.; Langer, R.; Mitchell, M. J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196.
- 7 Jain, R. K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664.
- 8 Hare, J. I.; Lammers, T.; Ashford, M. B.; Puri, S.; Storm, G.; Barry, S. T. Challenges and Strategies in Anti-cancer Nanomedicine Development: An Industry Perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38.
- 9 Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951.
- 10 Couvreur, P. Nanoparticles in Drug Delivery: Past, Present and Future. Adv. Drug Deliv. Rev. 2013, 65, 21–23.
- 11 Lammers, T.; Ferrari, M. The Success of Nanomedicine. Nano Today 2020, 31, 100853.
- 12 Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv. Mater. 2017, 29, 1700996.
- 13 Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638.
- 14 Sun, Q.; Ojha, T.; Kiessling, F.; Lammers, T.; Shi, Y. Enhancing Tumor Penetration of Nanomedicines. Biomacromolecules 2017, 18, 1449–1459.
- 15 Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, Challenges, and Future of Nanomedicine. Nano Today 2020, 35, 101008.
- 16 Farokhzad, O. C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20.
- 17 Chen, W. H.; Luo, G. F.; Zhang, X. Z. Recent Advances in Subcellular Targeted Cancer Therapy Based on Functional Materials. Adv. Mater. 2018, 31, e1802725.
- 18 Chen, W. H.; Luo, G. F.; Lei, Q.; Hong, S.; Qiu, W. X.; Liu, L. H.; Cheng, S. X.; Zhang, X. Z. Overcoming the Heat Endurance of Tumor Cells by Interfering with the Anaerobic Glycolysis Metabolism for Improved Photothermal Therapy. ACS Nano 2017, 11, 1419–1431.
- 19 Chen, W. H.; Luo, G. F.; Vázquez-González, M.; Cazelles, R.; Sohn, Y. S.; Nechushtai, R.; Mandel, Y.; Willner, I. Glucose-Responsive Metal-Organic-Framework Nanoparticles Act as “Smart” Sense-and-Treat Carriers. ACS Nano 2018, 12, 7538–7545.
- 20 Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y.; Wu, Q.; Li, L.; Huang, W. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy. Adv. Sci. 2021, 8, e2100876.
- 21 Ghosh, B.; Biswas, S. Polymeric Micelles in Cancer Therapy: State of the Art. J. Control. Release 2021, 332, 127–147.
- 22 He, H.; Liu, L.; Morin, E. E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines-Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019, 52, 2445–2461.
- 23 McGoron, A. J. Perspectives on the Future of Nanomedicine to Impact Patients: An Analysis of US Federal Funding and Interventional Clinical Trials. Bioconjug. Chem. 2020, 31, 436–447.
- 24 Yang, J.; Wang, X.; Wang, B.; Park, K.; Wooley, K.; Zhang, S. Challenging the Fundamental Conjectures in Nanoparticle Drug Delivery for Chemotherapy Treatment of Solid Cancers. Adv. Drug Deliv. Rev. 2022, 190, 114525.
- 25 Eble, J. A.; Niland, S. The Extracellular Matrix in Tumor Progression and Metastasis. Clin. Exp. Metastasis 2019, 36, 171–198.
- 26 Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K. J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120.
- 27 Li, H.; Fan, X.; Houghton, J. Tumor Microenvironment: The Role of the Tumor Stroma in Cancer. J. Cell. Biochem. 2007, 101, 805–815.
- 28 Yamauchi, M.; Barker, T. H.; Gibbons, D. L.; Kurie, J. M. The Fibrotic Tumor Stroma. J. Clin. Invest. 2018, 128, 16–25.
- 29 Zhang, J.; Liu, J. Tumor Stroma as Targets for Cancer Therapy. Pharmacol. Ther. 2013, 137, 200–215.
- 30 Chung, S. W.; Xie, Y.; Suk, J. S. Overcoming Physical Stromal Barriers to Cancer Immunotherapy. Drug Deliv. Transl. Res. 2021, 11, 2430–2447.
- 31 Liu, Y.; Zhou, J.; Li, Q.; Li, L.; Jia, Y.; Geng, F.; Zhou, J.; Yin, T. Tumor Microenvironment Remodeling-Based Penetration Strategies to Amplify Nanodrug Accessibility to Tumor Parenchyma. Adv. Drug Deliv. Rev. 2021, 172, 80–103.
- 32 Hu, J.; Yuan, X.; Wang, F.; Gao, H.; Liu, X.; Zhang, W. The Progress and Perspective of Strategies to Improve Tumor Penetration of Nanomedicines. Chin. Chem. Lett. 2021, 32, 1341–1347.
- 33 Liu, Z.; Ji, P.; Liu, H.; Yu, L.; Zhang, S. M.; Liu, P.; Zhang, X. Z.; Luo, G. F.; Shang, Z. FNIII14 Peptide-Enriched Membrane Nanocarrier to Disrupt Stromal Barriers through Reversing CAFs for Augmenting Drug Penetration in Tumors. Nano Lett. 2023, 23, 9963–9971.
- 34 Zhang, L.; Wang, Y.; Yang, Y.; Liu, Y.; Ruan, S.; Zhang, Q.; Tai, X.; Chen, J.; Xia, T.; Qiu, Y.; Gao, H.; He, Q. High Tumor Penetration of Paclitaxel Loaded pH Sensitive Cleavable Liposomes by Depletion of Tumor Collagen I in Breast Cancer. ACS Appl. Mater. Interfaces 2015, 7, 9691–9701.
- 35 Wang, S. B.; Chen, Z. X.; Gao, F.; Zhang, C.; Zou, M. Z.; Ye, J. J.; Zeng, X.; Zhang, X. Z. Remodeling Extracellular Matrix Based on Functional Covalent Organic Framework to Enhance Tumor Photodynamic Therapy. Biomaterials 2020, 234, 119772.
- 36 Lin, T. T.; Gao, D. Y.; Liu, Y. C.; Sung, Y. C.; Wan, D.; Liu, J. Y.; Chiang, T.; Wang, L.; Chen, Y. Development and Characterization of Sorafenib-Loaded PLGA Nanoparticles for the Systemic Treatment of Liver Fibrosis. J. Control. Release 2016, 221, 62–70.
- 37 Chakraborty, A.; Pinar, A. A.; Lam, M.; Bourke, J. E.; Royce, S. G.; Selomulya, C.; Samuel, C. S. Pulmonary Myeloid Cell Uptake of Biodegradable Nanoparticles Conjugated with an Anti-Fibrotic Agent Provides a Novel Strategy for Treating Chronic Allergic Airways Disease. Biomaterials 2021, 273, 120796.
- 38 Zhang, M.; Xu, H. Peptide-Assembled Nanoparticles Targeting Tumor Cells and Tumor Microenvironment for Cancer Therapy. Front. Chem. 2023, 11, 1115495.
- 39 Younis, M. A.; Khalil, I. A.; Elewa, Y. H. A.; Kon, Y.; Harashima, H. Ultra-Small Lipid Nanoparticles Encapsulating Sorafenib and Midkine-Sirna Selectively-Eradicate Sorafenib-Resistant Hepatocellular Carcinoma In Vivo. J. Control. Release 2021, 331, 335–349.
- 40 Zhang, Y.; Liu, Y.; Gao, X.; Li, X.; Niu, X.; Yuan, Z.; Wang, W. Near-Infrared-Light Induced Nanoparticles with Enhanced Tumor Tissue Penetration and Intelligent Drug Release. Acta Biomater. 2019, 90, 314–323.
- 41 Ikeda-Imafuku, M.; Wang, L. L. W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to Improve the EPR Effect: A Mechanistic Perspective and Clinical Translation. J. Control. Release 2022, 345, 512–536.
- 42 Dolor, A.; Szoka, F. C. Digesting a Path Forward: The Utility of Collagenase Tumor Treatment for Improved Drug Delivery. Mol. Pharmaceutics 2018, 15, 2069–2083.
- 43 Fu, Y.; Saraswat, A. L.; Monpara, J.; Patel, K. Stromal Disruption Facilitating Invasion of A ‘Nano-Arsenal’ into the Solid Tumor. Drug Discov. Today 2022, 27, 1132–1141.
- 44 Guan, X.; Chen, J.; Hu, Y.; Lin, L.; Sun, P.; Tian, H.; Chen, X. Highly Enhanced Cancer Immunotherapy by Combining Nanovaccine with Hyaluronidase. Biomaterials 2018, 171, 198–206.
- 45 Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.; Feng, L.; Liu, J.; Yang, G.; Chen, Q.; Liu, Z. Hyaluronidase to Enhance Nanoparticle-Based Photodynamic Tumor Therapy. Nano Lett. 2016, 16, 2512–2521.
- 46 Wang, H.; Han, X.; Dong, Z.; Xu, J.; Wang, J.; Liu, Z. Hyaluronidase with pH-responsive Dextran Modification as an Adjuvant Nanomedicine for Enhanced Photodynamic-Immunotherapy of Cancer. Adv. Funct. Mater. 2019, 29, 1902440.
- 47 Li, Y. J.; Wu, J. Y.; Hu, X. B.; Ding, T.; Tang, T.; Xiang, D. X. Biomimetic Liposome with Surface-Bound Elastase for Enhanced Tumor Penetration and Chemo-Immumotherapy. Adv. Healthc. Mater. 2021, 10, e2100794
- 48 Ikeda-Imafuku, M.; Gao, Y.; Shaha, S.; Wang, L. L. W.; Park, K. S.; Nakajima, M.; Adebowale, O.; Mitragotri, S. Extracellular Matrix Degrading Enzyme with Stroma-Targeting Peptides Enhance the Penetration of Liposomes into Tumors. J. Control. Release 2022, 352, 1093–1103.
- 49 Wu, D.; Chen, X.; Zhou, J.; Chen, Y.; Wan, T.; Wang, Y.; Lin, A.; Ruan, Y.; Chen, Z.; Song, X.; Fang, W.; Duan, H.; Ping, Y. A Synergistic Optical Strategy for Enhanced Deep-Tumor Penetration and Therapy in the Second Near-Infrared Window. Mater. Horiz. 2020, 7, 2929–2935.
- 50 Thomas, S. C.; Madaan, T.; Kamble, N. S.; Siddiqui, N. A.; Pauletti, G. M.; Kotagiri, N. Engineered Bacteria Enhance Immunotherapy and Targeted Therapy through Stromal Remodeling of Tumors. Adv. Healthc. Mater. 2021, 11, e2101487.
- 51 Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D. G.; Egeblad, M.; Evans, R. M.; Fearon, D.; Greten, F. R.; Hingorani, S. R.; Hunter, T.; Hynes, R. O.; Jain, R. K.; Janowitz, T.; Jorgensen, C.; Kimmelman, A. C.; Kolonin, M. G.; Maki, R. G.; Powers, R. S.; Puré, E.; Ramirez, D. C.; Scherz-Shouval, R.; Sherman, M. H.; Stewart, S.; Tlsty, T. D.; Tuveson, D. A.; Watt, F. M.; Weaver, V.; Weeraratna, A. T.; Werb, Z. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186.
- 52 Kobayashi, H.; Enomoto, A.; Woods, S. L.; Burt, A. D.; Takahashi, M.; Worthley, D. L. Cancer-Associated Fibroblasts in Gastrointestinal Cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 282–295.
- 53 Chen, Y.; McAndrews, K. M.; Kalluri, R. Clinical and Therapeutic Relevance of Cancer-Associated Fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804.
- 54 Lin, Y.; Cai, Q.; Chen, Y.; Shi, T.; Liu, W.; Mao, L.; Deng, B.; Ying, Z.; Gao, Y.; Luo, H.; Yang, X.; Huang, X.; Shi, Y.; He, R. CAFs Shape Myeloid-Derived Suppressor Cells to Promote Stemness of Intrahepatic Cholangiocarcinoma through 5-Lipoxygenase. Hepatology 2021, 75, 28–42.
- 55 Huo, M.; Zhou, J.; Wang, H.; Zheng, Y.; Tong, Y.; Zhou, J.; Liu, J.; Yin, T. A pHe Sensitive Nanodrug for Collaborative Penetration and Inhibition of Metastatic Tumors. J. Control. Release 2022, 352, 893–908.
- 56 Liu, Y.; Wu, X.; Chen, F.; Li, H.; Wang, T.; Liu, N.; Sun, K.; Zhou, G.; Tao, K. Modulating Cancer-Stroma Crosstalk by a Nanoparticle-Based Photodynamic Method to Pave the Way for Subsequent Therapies. Biomaterials 2022, 289, 121813.
- 57 Gong, X.; Li, J.; Xu, X.; Wu, Y.; Lei, Y.; Liu, H.; Qian, X.; Li, Y.; Zhang, Z. Microvesicle-Inspired Oxygen-Delivering Nanosystem Potentiates Radiotherapy-Mediated Modulation of Tumor Stroma and Antitumor Immunity. Biomaterials 2022, 290, 121855.
- 58 Xu, H.; Hu, M.; Liu, M.; An, S.; Guan, K.; Wang, M.; Li, L.; Zhang, J.; Li, J.; Huang, L. Nano-Puerarin Regulates Tumor Microenvironment and Facilitates Chemo- and Immunotherapy in Murine Triple Negative Breast Cancer Model. Biomaterials 2020, 235, 119769.
- 59 Ji, T.; Ding, Y.; Zhao, Y.; Wang, J.; Qin, H.; Liu, X.; Lang, J.; Zhao, R.; Zhang, Y.; Shi, J.; Tao, N.; Qin, Z.; Nie, G. Peptide Assembly Integration of Fibroblast-Targeting and Cell-Penetration Features for Enhanced Antitumor Drug Delivery. Adv. Mater. 2015, 27, 1865–1873.
- 60 Feng, X.; Dixon, H.; Glen-Ravenhill, H.; Karaosmanoglu, S.; Li, Q.; Yan, L.; Chen, X. Smart Nanotechnologies to Target Tumor with Deep Penetration Depth for Efficient Cancer Treatment and Imaging. Adv. Ther. 2019, 2, 1900093.
- 61 Niu, Y.; Zhu, J.; Li, Y.; Shi, H.; Gong, Y.; Li, R.; Huo, Q.; Ma, T.; Liu, Y. Size Shrinkable Drug Delivery Nanosystems and Priming the Tumor Microenvironment for Deep Intratumoral Penetration of Nanoparticles. J. Control. Release 2018, 277, 35–47.
- 62 Nichols, J. W.; Sakurai, Y.; Harashima, H.; Bae, Y. H. Nano-Sized Drug Carriers: Extravasation, Intratumoral Distribution, and Their Modeling. J. Control. Release 2017, 267, 31–46.
- 63 Liu, J.; Chen, Q.; Zhu, W.; Yi, X.; Yang, Y.; Dong, Z.; Liu, Z. Nanoscale-Coordination-Polymer-Shelled Manganese Dioxide Composite Nanoparticles: A Multistage Redox/pH/H2O2-Responsive Cancer Theranostic Nanoplatform. Adv. Funct. Mater. 2017, 27, 1605926.
- 64 Vlashi, E.; Kelderhouse, L. E.; Sturgis, J. E.; Low, P. S. Effect of Folate-Targeted Nanoparticle Size on Their Rates of Penetration into Solid Tumors. ACS Nano 2013, 7, 8573–8582.
- 65 Chen, F.; Ma, K.; Madajewski, B.; Zhuang, L.; Zhang, L.; Rickert, K.; Marelli, M.; Yoo, B.; Turker, M. Z.; Overholtzer, M.; Quinn, T. P.; Gonen, M.; Zanzonico, P.; Tuesca, A.; Bowen, M. A.; Norton, L.; Subramony, J. A.; Wiesner, U.; Bradbury, M. S. Ultrasmall Targeted Nanoparticles with Engineered Antibody Fragments for Imaging Detection of HER2-Overexpressing Breast Cancer. Nat. Commun. 2018, 9, 4141.
- 66 Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M. R.; Miyazono, K.; Uesaka, M.; Nishiyama, N.; Kataoka, K. Accumulation of Sub-100 nm Polymeric Micelles in Poorly Permeable Tumours Depends on Size. Nat. Nanotechnol. 2011, 6, 815–823.
- 67 You, J.; Zhang, R.; Xiong, C.; Zhong, M.; Melancon, M.; Gupta, S.; Nick, A. M.; Sood, A. K.; Li, C. Effective Photothermal Chemotherapy Using Doxorubicin-Loaded Gold Nanospheres That Target EphB4 Receptors in Tumors. Cancer Res. 2012, 72, 4777–4786.
- 68 Li, H. J.; Du, J. Z.; Du, X. J.; Xu, C. F.; Sun, C. Y.; Wang, H. X.; Cao, Z. T.; Yang, X. Z.; Zhu, Y. H.; Nie, S.; Wang, J. Stimuli-Responsive Clustered Nanoparticles for Improved Tumor Penetration and Therapeutic Efficacy. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 4164–4169.
- 69 Dai, L.; Li, K.; Li, M.; Zhao, X.; Luo, Z.; Lu, L.; Luo, Y.; Cai, K. Size/Charge Changeable Acidity-Responsive Micelleplex for Photodynamic-Improved PD-L1 Immunotherapy with Enhanced Tumor Penetration. Adv. Funct. Mater. 2018, 28, 1707249.
- 70 Hao, Q.; Wang, Z.; Zhao, W.; Wen, L.; Wang, W.; Lu, S.; Xing, D.; Zhan, M.; Hu, X. Dual-Responsive Polyprodrug Nanoparticles with Cascade-Enhanced Magnetic Resonance Signals for Deep-Penetration Drug Release in Tumor Therapy. ACS Appl. Mater. Interfaces 2020, 12, 49489–49501.
- 71 Hua, L.; Wang, Z.; Zhao, L.; Mao, H.; Wang, G.; Zhang, K.; Liu, X.; Wu, D.; Zheng, Y.; Lu, J.; Yu, R.; Liu, H. Hypoxia-Responsive Lipid-Poly-(Hypoxic Radiosensitized Polyprodrug) Nanoparticles for Glioma Chemo- and Radiotherapy. Theranostics 2018, 8, 5088–5105.
- 72 He, P.; Lei, Q.; Yang, B.; Shang, T.; Shi, J.; Ouyang, Q.; Wang, W.; Xue, L.; Kong, F.; Li, Z.; Huang, J.; Liu, L.; Guo, J.; Brinker, C. J.; Liu, K.; Zhu, W. Dual-Stage Irradiation of Size-Switchable Albumin Nanocluster for Cascaded Tumor Enhanced Penetration and Photothermal Therapy. ACS Nano 2022, 16, 13919–13932.
- 73 Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-Triggered Size Shrink and Laser-Enhanced NO Release Nanoparticles for Deep Tumor Penetration and Combination therapy. Biomaterials 2018, 168, 64–75.
- 74 Tong, R.; Hemmati, H. D.; Langer, R.; Kohane, D. S. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery. J. Am. Chem. Soc. 2012, 134, 8848–8855.
- 75 Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 2017, 29, 1701170.
- 76 Zhang, J.; Yuan, Z. F.; Wang, Y.; Chen, W. H.; Luo, G. F.; Cheng, S. X.; Zhuo, R. X.; Zhang, X. Z. Multifunctional Envelope-Type Mesoporous Silica Nanoparticles for Tumor-Triggered Targeting Drug Delivery. J. Am. Chem. Soc. 2013, 135, 5068–5073.
- 77 Ruan, S.; Cao, X.; Cun, X.; Hu, G.; Zhou, Y.; Zhang, Y.; Lu, L.; He, Q.; Gao, H. Matrix Metalloproteinase-Sensitive Size-Shrinkable Nanoparticles for Deep Tumor Penetration and pH Triggered Doxorubicin Release. Biomaterials 2015, 60, 100-110.
- 78 Cun, X.; Li, M.; Wang, S.; Wang, Y.; Wang, J.; Lu, Z.; Yang, R.; Tang, X.; Zhang, Z.; He, Q. A Size Switchable Nanoplatform for Targeting the Tumor Microenvironment and Deep Tumor Penetration. Nanoscale 2018, 10, 9935–9948.
- 79 Kim, J.; Jo, C.; Lim, W. G.; Jung, S.; Lee, Y. M.; Lim, J.; Lee, H.; Lee, J.; Kim, W. J. Programmed Nanoparticle-Loaded Nanoparticles for Deep-Penetrating 3D Cancer Therapy. Adv. Mater. 2018, 30, 1707557.
- 80 Lei, Q.; Wang, S. B.; Hu, J. J.; Lin, Y. X.; Zhu, C. H.; Rong, L.; Zhang, X. Z. Stimuli-Responsive “Cluster Bomb” for Programmed Tumor Therapy. ACS Nano 2017, 11, 7201–7214.
- 81 Du, J. Z.; Du, X. J.; Mao, C. Q.; Wang, J. Tailor-Made Dual pH-Sensitive Polymer-Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J. Am. Chem. Soc. 2011, 133, 17560–17563.
- 82 Li, L.; Sun, W.; Zhong, J.; Yang, Q.; Zhu, X.; Zhou, Z.; Zhang, Z.; Huang, Y. Multistage Nanovehicle Delivery System Based on Stepwise Size Reduction and Charge Reversal for Programmed Nuclear Targeting of Systemically Administered Anticancer Drugs. Adv. Funct. Mater. 2015, 25, 4101–4113.
- 83 Nguyen, L. N. M.; Lin, Z. P.; Sindhwani, S.; MacMillan, P.; Mladjenovic, S. M.; Stordy, B.; Ngo, W.; Chan, W. C. W. The Exit of Nanoparticles from Solid Tumours. Nat. Mater. 2023, 22, 1261–1272.
- 84 Ouyang, B.; Poon, W.; Zhang, Y. N.; Lin, Z. P.; Kingston, B. R.; Tavares, A. J.; Zhang, Y.; Chen, J.; Valic, M. S.; Syed, A. M.; MacMillan, P.; Couture-Senécal, J.; Zheng, G.; Chan, W. C. W. The Dose Threshold for Nanoparticle Tumour Delivery. Nat. Mater. 2020, 19, 1362–1371.
- 85 Li, J.; Wang, H.; Wang, Y.; Gong, X.; Xu, X.; Sha, X.; Zhang, A.; Zhang, Z.; Li, Y. Tumor-Activated Size-Enlargeable Bioinspired Lipoproteins Access Cancer Cells in Tumor to Elicit Anti-Tumor Immune Responses. Adv. Mater. 2020, 32, 2002380.
- 86 Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y. A.; Yang, L.; Mao, H. Exerting Enhanced Permeability and Retention Effect Driven Delivery by Ultrafine Iron Oxide Nanoparticles with T1–T2 Switchable Magnetic Resonance Imaging Contrast. ACS Nano 2017, 11, 4582–4592.
- 87 Chen, J.; Jiang, Z.; Zhang, Y. S.; Ding, J.; Chen, X. Smart Transformable Nanoparticles for Enhanced Tumor Theranostics. Appl. Phys. Rev. 2021, 8, 041321.
- 88 Hua, S.; He, J.; Zhang, F.; Yu, J.; Zhang, W.; Gao, L.; Li, Y.; Zhou, M. Multistage-Responsive Clustered Nanosystem to Improve Tumor Accumulation and Penetration for Photothermal/Enhanced Radiation Synergistic Therapy. Biomaterials 2021, 268, 120590.
- 89 Wu, W.; Li, S.; Lin, Z.; Li, J. pH-Sensitive Nanocarriers for Enhanced Tumor Retention and Rapid Intracellular Drug Release. J. Control. Release 2015, 213, e111–e112.
- 90 Chen, Y.; Zhang, X. H.; Cheng, D. B.; Zhang, Y.; Liu, Y.; Ji, L.; Guo, R.; Chen, H.; Ren, X. K.; Chen, Z.; Qiao, Z. Y.; Wang, H. Near-Infrared Laser-Triggered in situ Dimorphic Transformation of BF2-Azadipyrromethene Nanoaggregates for Enhanced Solid Tumor Penetration. ACS Nano 2020, 14, 3640–3650.
- 91 Wang, Y.; Wang, D.; Fu, Q.; Liu, D.; Ma, Y.; Racette, K.; He, Z.; Liu, F. Shape-Controlled Paclitaxel Nanoparticles with Multiple Morphologies: Rod-Shaped, Worm-Like, Spherical, and Fingerprint-Like. Mol. Pharmaceutics 2014, 11, 3766–3771.
- 92 Kinnear, C.; Moore, T. L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521.
- 93 Han, K.; Zhang, J.; Zhang, W.; Wang, S.; Xu, L.; Zhang, C.; Zhang, X.; Han, H. Tumor-Triggered Geometrical Shape Switch of Chimeric Peptide for Enhanced in Vivo Tumor Internalization and Photodynamic Therapy. ACS Nano 2017, 11, 3178–3188.
- 94 Wang, W.; Gaus, K.; Tilley, R. D.; Gooding, J. J. The Impact of Nanoparticle Shape on Cellular Internalisation and Transport: What Do the Different Analysis Methods Tell Us? Mater. Horiz. 2019, 6, 1538–1547.
- 95 Moyer, T. J.; Kassam, H. A.; Bahnson, E. S. M.; Morgan, C. E.; Tantakitti, F.; Chew, T. L.; Kibbe, M. R.; Stupp, S. I. Shape-Dependent Targeting of Injured Blood Vessels by Peptide Amphiphile Supramolecular Nanostructures. Small 2015, 11, 2750–2755.
- 96 Chithrani, B. D.; Ghazani, A. A.; Chan, W. C. W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668.
- 97 Chauhan, V. P.; Popović, Z.; Chen, O.; Cui, J.; Fukumura, D.; Bawendi, M. G.; Jain, R. K. Fluorescent Nanorods and Nanospheres for Real-Time In Vivo Probing of Nanoparticle Shape-Dependent Tumor Penetration. Angew. Chem. Int. Ed. 2011, 50, 11417–11420.
- 98 Dasgupta, S.; Auth, T.; Gompper, G. Shape and Orientation Matter for the Cellular Uptake of Nonspherical Particles. Nano Lett. 2014, 14, 687–693.
- 99 Wang, Z.; Wang, Y.; Jia, X.; Han, Q.; Qian, Y.; Li, Q.; Xiang, J.; Wang, Q.; Hu, Z.; Wang, W. MMP-2-Controlled Transforming Micelles for Heterogeneic Targeting and Programmable Cancer Therapy. Theranostics 2019, 9, 1728–1740.
- 100 Zhou, Y.; Li, Q.; Wu, Y.; Li, X.; Zhou, Y.; Wang, Z.; Liang, H.; Ding, F.; Hong, S.; Steinmetz, N. F.; Cai, H. Molecularly Stimuli-Responsive Self-Assembled Peptide Nanoparticles for Targeted Imaging and Therapy. ACS Nano 2023, 17, 8004–8025.
- 101 Sun, B.; Chang, R.; Cao, S.; Yuan, C.; Zhao, L.; Yang, H.; Li, J.; Yan, X.; van Hest, J. C. M. Acid-Activatable Transmorphic Peptide-Based Nanomaterials for Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 20582–20588.
- 102 Li, M.; Ning, Y.; Chen, J.; Duan, X.; Song, N.; Ding, D.; Su, X.; Yu, Z. Proline Isomerization-Regulated Tumor Microenvironment-Adaptable Self-Assembly of Peptides for Enhanced Therapeutic Efficacy. Nano Lett. 2019, 19, 7965–7976.
- 103 Song, N.; Zhou, Z.; Song, Y.; Li, M.; Yu, X.; Hu, B.; Yu, Z. In Situ Oxidation-Regulated Self-Assembly of Peptides into Transformable Scaffolds for Cascade Therapy. Nano Today 2021, 38, 101198.
- 104 Poon, C.; McMahon, D.; Hynynen, K. Noninvasive and Targeted Delivery of Therapeutics to the Brain Using Focused Ultrasound. Neuropharmacology 2017, 120, 20–37.
- 105 Lee, S.; Han, H.; Koo, H.; Na, J. H.; Yoon, H. Y.; Lee, K. E.; Lee, H.; Kim, H.; Kwon, I. C.; Kim, K. Extracellular Matrix Remodeling In Vivo for Enhancing Tumor-Targeting Efficiency of Nanoparticle Drug Carriers Using the Pulsed High Intensity Focused Ultrasound. J. Control. Release 2017, 263, 68–78.
- 106 Tham, H. P.; Xu, K.; Lim, W. Q.; Chen, H.; Zheng, M.; Thng, T. G. S.; Venkatraman, S. S.; Xu, C.; Zhao, Y. Microneedle-Assisted Topical Delivery of Photodynamically Active Mesoporous Formulation for Combination Therapy of Deep-Seated Melanoma. ACS Nano 2018, 12, 11936–11948.
- 107 Shamsi, M.; Sedaghatkish, A.; Dejam, M.; Saghafian, M.; Mohammadi, M.; Sanati-Nezhad, A. Magnetically Assisted Intraperitoneal Drug Delivery for Cancer Chemotherapy. Drug Deliv. 2018, 25, 846–861.
- 108 Guo, X.; Wu, Z.; Li, W.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Zhu, X.; Du, Y. P.; Jin, Y.; Du, Y.; You, J. Appropriate Size of Magnetic Nanoparticles for Various Bioapplications in Cancer Diagnostics and Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3092–3106.
- 109 Wang, Y.; Li, Y.; Yan, K.; Shen, L.; Yang, W.; Gong, J.; Ding, K. Clinical Study of Ultrasound and Microbubbles for Enhancing Chemotherapeutic Sensitivity of Malignant Tumors in Digestive System. Chin. J. Cancer Res. 2018, 30, 553–563.
- 110 Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y.; Capelle, L.; Cornu, P.; Sanson, M.; Hoang-Xuan, K.; Delattre, J. Y.; Idbaih, A. Clinical Trial of Blood-Brain Barrier Disruption by Pulsed Ultrasound. Sci. Transl. Med. 2016, 8, 343re2.
- 111 Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; De Rycke, Y.; Trosch, C.; Capelle, L.; Sanson, M.; Hoang-Xuan, K.; Dehais, C.; Houillier, C.; Laigle-Donadey, F.; Mathon, B.; André, A.; Lafon, C.; Chapelon, J. Y.; Delattre, J. Y.; Carpentier, A. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801.
- 112 Theek, B.; Baues, M.; Ojha, T.; Möckel, D.; Veettil, S. K.; Steitz, J.; van Bloois, L.; Storm, G.; Kiessling, F.; Lammers, T. Sonoporation Enhances Liposome Accumulation and Penetration in Tumors with Low EPR. J. Control. Release 2016, 231, 77–85.
- 113 Sulheim, E.; Hanson, I.; Snipstad, S.; Vikedal, K.; Mørch, Y.; Boucher, Y.; Davies, C. d. L. Sonopermeation with Nanoparticle-Stabilized Microbubbles Reduces Solid Stress and Improves Nanomedicine Delivery to Tumors. Adv. Ther. 2021, 4, 2100147.
- 114 Kulkarni, S.; Ramaswamy, B.; Horton, E.; Gangapuram, S.; Nacev, A.; Depireux, D.; Shimoji, M.; Shapiro, B. Quantifying the Motion of Magnetic Particles in Excised Tissue: Effect of Particle Properties and Applied Magnetic Field. J. Magn. Magn. Mater. 2015, 393, 243–252.
- 115 Veiseh, O.; Gunn, J. W.; Zhang, M. Design and Fabrication of Magnetic Nanoparticles for Targeted Drug Delivery and Imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304.
- 116 Zhou, Z.; Shen, Z.; Chen, X. Tale of Two Magnets: An Advanced Magnetic Targeting System. ACS Nano 2019, 14, 7–11.
- 117 Belyanina, I. V.; Zamay, T. N.; Zamay, G. S.; Zamay, S. S.; Kolovskaya, O. S.; Ivanchenko, T. I.; Denisenko, V. V.; Kirichenko, A. K.; Glazyrin, Y. E.; Garanzha, I. V.; Grigorieva, V. V.; Shabanov, A. V.; Veprintsev, D. V.; Sokolov, A. E.; Sadovskii, V. M.; Gargaun, A.; Berezovski, M. V.; Kichkailo, A. S. In Vivo Cancer Cells Elimination Guided by Aptamer-Functionalized Gold-Coated Magnetic Nanoparticles and Controlled with Low Frequency Alternating Magnetic Field. Theranostics 2017, 7, 3326–3337.
- 118 Su, Y. L.; Fang, J. H.; Liao, C. Y.; Lin, C. T.; Li, Y. T.; Hu, S. H. Targeted Mesoporous Iron Oxide Nanoparticles-Encapsulated Perfluorohexane and A Hydrophobic Drug for Deep Tumor Penetration and Therapy. Theranostics 2015, 5, 1233–1248.
- 119 Liu, J. F.; Lan, Z.; Ferrari, C.; Stein, J. M.; Higbee-Dempsey, E.; Yan, L.; Amirshaghaghi, A.; Cheng, Z.; Issadore, D.; Tsourkas, A. Use of Oppositely Polarized External Magnets to Improve the Accumulation and Penetration of Magnetic Nanocarriers into Solid Tumors. ACS Nano 2019, 14, 142-152.
- 120 Zhang, Z.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.; Wu, X.; Zhao, Y.; Chen, C. Near Infrared Laser-Induced Targeted Cancer Therapy Using Thermoresponsive Polymer Encapsulated Gold Nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326.
- 121 Luo, G. F.; Chen, W. H.; Lei, Q.; Qiu, W. X.; Liu, Y. X.; Cheng, Y. J.; Zhang, X. Z. A Triple-Collaborative Strategy for High-Performance Tumor Therapy by Multifunctional Mesoporous Silica-Coated Gold Nanorods. Adv. Funct. Mater. 2016, 26, 4339–4350.
- 122 Tan, T.; Hu, H.; Wang, H.; Li, J.; Wang, Z.; Wang, J.; Wang, S.; Zhang, Z.; Li, Y. Bioinspired Lipoproteins-Mediated Photothermia Remodels Tumor Stroma to Improve Cancer Cell Accessibility of Second Nanoparticles. Nat. Commun. 2019, 10, 3322.
- 123 Esteban-Fernández de Ávila, B.; Gao, W.; Karshalev, E.; Zhang, L.; Wang, J. Cell-Like Micromotors. Acc. Chem. Res. 2018, 51, 1901–1910.
- 124 Magdanz, V.; Medina-Sánchez, M.; Schwarz, L.; Xu, H.; Elgeti, J.; Schmidt, O. G. Spermatozoa as Functional Components of Robotic Microswimmers. Adv. Mater. 2017, 29, 1606301.
- 125 Yasa, O.; Erkoc, P.; Alapan, Y.; Sitti, M. Microalga-Powered Microswimmers toward Active Cargo Delivery. Adv. Mater. 2018, 30, 1804130.
- 126 Chen, Q. W.; Qiao, J. Y.; Liu, X. H.; Zhang, C.; Zhang, X. Z. Customized Materials-Assisted Microorganisms in Tumor Therapeutics. Chem. Soc. Rev. 2021, 50, 12576–12615.
- 127 Luo, G. F.; Chen, W. H.; Zeng, X.; Zhang, X. Z. Cell Primitive-Based Biomimetic Functional Materials for Enhanced Cancer Therapy. Chem. Soc. Rev. 2021, 50, 945–985.
- 128 Krell, T.; Lacal, J.; Muñoz-Martínez, F.; Reyes-Darias, J. A.; Cadirci, B. H.; García-Fontana, C.; Ramos, J. L. Diversity at Its Best: Bacterial Taxis. Environ. Microbiol. 2010, 13, 1115–1124.
- 129 Martel, S. Bacterial Microsystems and Microrobots. Biomed. Microdevices 2012, 14, 1033–1045.
- 130 Lee, C. H. Engineering Bacteria toward Tumor Targeting for Cancer Treatment: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2011, 93, 517–523.
- 131 Forbes, N. S. Engineering the Perfect (Bacterial) Cancer Therapy. Nat. Rev. Cancer 2010, 10, 785–794.
- 132 Wang, J. W.; Chen, Q. W.; Luo, G. F.; Han, Z. Y.; Song, W. F.; Yang, J.; Chen, W. H.; Zhang, X. Z. A Self-Driven Bioreactor Based on Bacterium-Metal-Organic Framework Biohybrids for Boosting Chemotherapy via Cyclic Lactate Catabolism. ACS Nano 2021, 15, 17870–17884.
- 133 Zhang, S. M.; Jin, X. K.; Luo, G. F.; Liang, J. L.; Wang, Y. Z.; Wang, J. W.; Meng, R.; Lin, Y. T.; Chen, W. H.; Zhang, X. Z. An Engineered Bacterium-Based Lactate Bioconsumer for Regulating Immunosuppressive Tumor Microenvironment to Potentiate Antitumor Immunity. ACS Mater. Lett. 2023, 5, 2785–2798.
- 134 Yin, T.; Diao, Z.; Blum, N. T.; Qiu, L.; Ma, A.; Huang, P. Engineering Bacteria and Bionic Bacterial Derivatives with Nanoparticles for Cancer Therapy. Small 2021, 18, 2104643.
- 135 Luo, C. H.; Huang, C. T.; Su, C. H.; Yeh, C. S. Bacteria-Mediated Hypoxia-Specific Delivery of Nanoparticles for Tumors Imaging and Therapy. Nano Lett. 2016, 16, 3493–3499.
- 136 Moreno, V. M.; Álvarez, E.; Izquierdo-Barba, I.; Baeza, A.; Serrano-López, J.; Vallet-Regí, M. Bacteria as Nanoparticles Carrier for Enhancing Penetration in a Tumoral Matrix Model. Adv. Mater. Interfaces 2020, 7, 1901942.
- 137 Schuerle, S.; Soleimany, A. P.; Yeh, T.; Anand, G. M.; Häberli, M.; Fleming, H. E.; Mirkhani, N.; Qiu, F.; Hauert, S.; Wang, X.; Nelson, B. J.; Bhatia, S. N. Synthetic and Living Micropropellers for Convection-Enhanced Nanoparticle Transport. Sci. Adv. 2019, 5, eaav4803.
- 138 Felfoul, O.; Mohammadi, M.; Taherkhani, S.; de Lanauze, D.; Xu, Y. Z.; Loghin, D.; Essa, S.; Jancik, S.; Houle, D.; Lafleur, M.; Gaboury, L.; Tabrizian, M.; Kaou, N.; Atkin, M.; Vuong, T.; Batist, G.; Beauchemin, N.; Radzioch, D.; Martel, S. Magneto-Aerotactic Bacteria Deliver Drug-Containing Nanoliposomes to Tumour Hypoxic Regions. Nat. Nanotechnol. 2016, 11, 941–947.
- 139 Chen, W.; Jiang, R.; Sun, X.; Chen, S.; Liu, X.; Fu, M.; Yan, X.; Ma, X. Self-Fueled Janus Nanomotors as Active Drug Carriers for Propulsion Behavior-Reinforced Permeability and Accumulation at the Tumor Site. Chem. Mater. 2022, 34, 7543–7552.
- 140 Ji, X.; Yang, H.; Liu, W.; Ma, Y.; Wu, J.; Zong, X.; Yuan, P.; Chen, X.; Yang, C.; Li, X.; Lin, H.; Xue, W.; Dai, J. Multifunctional Parachute-like Nanomotors for Enhanced Skin Penetration and Synergistic Antifungal Therapy. ACS Nano 2021, 15, 14218–14228.
- 141 Chen, Z.; Xia, T.; Zhang, Z.; Xie, S.; Wang, T.; Li, X. Enzyme-Powered Janus Nanomotors Launched from Intratumoral Depots to Address Drug Delivery Barriers. Chem. Eng. J. 2019, 375, 122109.
- 142 Zheng, J.; Wang, W.; Gao, X.; Zhao, S.; Chen, W.; Li, J.; Liu, Y. N. Cascade Catalytically Released Nitric Oxide-Driven Nanomotor with Enhanced Penetration for Antibiofilm. Small 2022, 18, 2205252.
- 143 Zhang, Y.; Zhang, K.; Yang, H.; Hao, Y.; Zhang, J.; Zhao, W.; Zhang, S.; Ma, S.; Mao, C. Highly Penetrable Drug-Loaded Nanomotors for Photothermal-Enhanced Ferroptosis Treatment of Tumor. ACS Appl. Mater. Interfaces 2023, 15, 14099–14110.
- 144 Chen, H.; Shi, T.; Wang, Y.; Liu, Z.; Liu, F.; Zhang, H.; Wang, X.; Miao, Z.; Liu, B.; Wan, M.; Mao, C.; Wei, J. Deep Penetration of Nanolevel Drugs and Micrometer-Level T Cells Promoted by Nanomotors for Cancer Immunochemotherapy. J. Am. Chem. Soc. 2021, 143, 12025–12037.
- 145 Gao, C.; Wang, Y.; Ye, Z.; Lin, Z.; Ma, X.; He, Q. Biomedical Micro-/Nanomotors: From Overcoming Biological Barriers to In Vivo Imaging. Adv. Mater. 2020, 33, 2000512.
- 146 Zheng, S.; Wang, Y.; Pan, S.; Ma, E.; Jin, S.; Jiao, M.; Wang, W.; Li, J.; Xu, K.; Wang, H. Biocompatible Nanomotors as Active Diagnostic Imaging Agents for Enhanced Magnetic Resonance Imaging of Tumor Tissues In Vivo. Adv. Funct. Mater. 2021, 31, 2100936.
- 147 Zhong, H.; Zhang, Z.; Zhou, Y.; Wu, L.; Ke, P.; Lu, Y.; Dai, Q.; Bao, X.; Xia, Y.; Yang, Q.; Tan, X.; Wei, Q.; Xu, W.; Han, M.; Ma, L. Pt/DOX Nanomotors Enhance Penetration in the Deep Tumor by Positive Chemotaxis. ACS Appl. Mater. Interfaces 2022, 14, 38172–38184.
- 148
Wan, M. M.; Chen, H.; Da Wang, Z.; Liu, Z. Y.; Yu, Y. Q.; Li, L.; Miao, Z. Y.; Wang, X. W.; Wang, Q.; Mao, C.; Shen, J.; Wei, J. Nitric Oxide-Driven Nanomotor for Deep Tissue Penetration and Multidrug Resistance Reversal in Cancer Therapy. Adv. Sci. 2020, 8, 2002525.
10.1002/advs.202002525 Google Scholar
- 149 Cole, C.; Qiao, J.; Kottke, T.; Diaz, R. M.; Ahmed, A.; Sanchez-Perez, L.; Brunn, G.; Thompson, J.; Chester, J.; Vile, R. G. Tumor-Targeted, Systemic Delivery of Therapeutic Viral Vectors using Hitchhiking on Antigen-Specific T Cells. Nat. Med. 2005, 11, 1073–1081.
- 150 Zheng, L.; Hu, X.; Wu, H.; Mo, L.; Xie, S.; Li, J.; Peng, C.; Xu, S.; Qiu, L.; Tan, W. In Vivo Monocyte/Macrophage-Hitchhiked Intratumoral Accumulation of Nanomedicines for Enhanced Tumor Therapy. J. Am. Chem. Soc. 2019, 142, 382–391.
- 151 Qi, J.; Jin, F.; You, Y.; Du, Y.; Liu, D.; Xu, X.; Wang, J.; Zhu, L.; Chen, M.; Shu, G.; Wu, L.; Ji, J.; Du, Y. Synergistic Effect of Tumor Chemo-Immunotherapy Induced by Leukocyte-Hitchhiking Thermal-Sensitive Micelles. Nat. Commun. 2021, 12, 4755.
- 152 Gao, C.; Wang, Q.; Li, J.; Kwong, C. H. T.; Wei, J.; Xie, B.; Lu, S.; Lee, S.; M. Y.; Wang, R. In Vivo Hitchhiking of Immune Cells by Intracellular Self-Assembly of Bacteria-Mimetic Nanomedicine for Targeted Therapy of Melanoma. Sci. Adv. 2022, 8, eabn1805.
- 153 Padmakumar, A.; Koyande, N. P.; Rengan, A. K. The Role of Hitchhiking in Cancer Therapeutics-A Review. Adv. Therap. 2022, 5, 2200042.
- 154 Wang, X.; Chen, D.; Huang, K.; Li, M.; Zhan, C.; Dong, Z.; Deng, T.; Ren, K.; Qiu, Y.; Zhang, Z.; He, Q. Albumin-Hitchhiking Drug Delivery to Tumor-Draining Lymph Nodes Precisely Boosts Tumor-Specific Immunity through Autophagy Modulation of Immune Cells. Adv. Mater. 2023, 35, e2211055.
- 155 Chen, K. H.; Nguyen, N.; Huang, T. Y.; Lin, Y. J.; Yu, Y. T.; Song, H. L.; Wang, J. T.; Nguyen, V. K.; Chen, H. L.; Chu, L. A.; Chiang, H. H. K.; Sung, H. W. Macrophage-Hitchhiked Orally Administered β-Glucans- Functionalized Nanoparticles as “Precision-Guided Stealth Missiles” for Targeted Pancreatic Cancer Therapy. Adv. Mater. 2023, 35, e2304735.
- 156 Kuang, J.; Rao, Z. Y.; Zheng, D. W.; Kuang, D.; Huang, Q. X.; Pan, T.; Li, H.; Zeng, X.; Zhang, X. Z. Nanoparticles Hitchhike on Monocytes for Glioblastoma Treatment after Low-Dose Radiotherapy. ACS Nano 2023, 17, 13333–13347.
- 157 Li, H.; Pei, P.; He, Q.; Dong, X.; Zhang, C.; Shen, W.; Chen, H.; Hu, L.; Tao, Y.; Yang, K. Nanozyme-Coated Bacteria Hitchhike on CD11b+ Immune Cells to Boost Tumor Radio-Immunotherapy. Adv. Mater. 2023, DOI: https://doi.org/10.1002/adma.202309332.
- 158 Wang, Y.; Sun, S. K.; Liu, Y.; Zhang, Z. Advanced Hitchhiking Nanomaterials for Biomedical Applications. Theranostics 2023, 13, 4781–4801.
- 159 Wang, Q.; Liang, Q.; Dou, J.; Zhou, H.; Zeng, C.; Pan, H.; Shen, Y.; Li, Q.; Liu, Y.; Leong, D. T.; Jiang, W.; Wang, Y. Breaking Through the Basement Membrane Barrier to Improve Nanotherapeutic Delivery to Tumours. Nat. Nanotechnol. 2023, DOI: https://doi.org/10.1038/s41565-023-01498-w.
- 160 Wiley, D. T.; Webster, P.; Gale, A.; Davis, M. E. Transcytosis and Brain Uptake of Transferrin-Containing Nanoparticles by Tuning Avidity to Transferrin Receptor. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 8662–8667.
- 161 Liu, Y.; Huo, Y.; Yao, L.; Xu, Y.; Meng, F.; Li, H.; Sun, K.; Zhou, G.; Kohane, D. S.; Tao, K. Transcytosis of Nanomedicine for Tumor Penetration. Nano Lett. 2019, 19, 8010–8020.
- 162 Wu, J.; Guo, Z.; Ni, W.; Feng, Y.; Guo, X.; Meng, M.; Yuan, Y.; Lin, L.; Chen, J.; Tian, H.; Chen, X. Novel Cocktail Therapy Based on a Nanocarrier with an Efficient Transcytosis Property Reverses the Dynamically Deteriorating Tumor Microenvironment for Enhanced Immunotherapy. Nano Lett. 2022, 22, 7220–7229.
- 163 Fan, W.; Xiang, J.; Wei, Q.; Tang, Y.; Piao, Y.; Shao, S.; Zhou, Z.; Tang, J.; Li, Z. C.; Shen, Y. Role of Micelle Size in Cell Transcytosis-Based Tumor Extravasation, Infiltration, and Treatment Efficacy. Nano Lett. 2023, 23, 3904–3912.
- 164 Wang, L.; Dou, J.; Jiang, W.; Wang, Q.; Liu, Y.; Liu, H.; Wang, Y. Enhanced Intracellular Transcytosis of Nanoparticles by Degrading Extracellular Matrix for Deep Tissue Radiotherapy of Pancreatic Adenocarcinoma. Nano Lett. 2022, 22, 6877–6887.
- 165 Pandit, S.; Dutta, D.; Nie, S. Active Transcytosis and New Opportunities for Cancer Nanomedicine. Nat. Mater. 2020, 19, 478–480.
- 166 Morad, G.; Carman, C. V.; Hagedorn, E. J.; Perlin, J. R.; Zon, L. I.; Mustafaoglu, N.; Park, T. E.; Ingber, D. E.; Daisy, C. C.; Moses, M. A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865.
- 167 Sindhwani, S.; Syed, A. M.; Ngai, J.; Kingston, B. R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N. U.; Hoang, T.; Wu, J. L. Y.; Wilhelm, S.; Zilman, A.; Gadde, S.; Sulaiman, A.; Ouyang, B.; Lin, Z.; Wang, L.; Egeblad, M.; Chan, W. C. W. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575.
- 168 Xiao, W.; Wang, Y.; Zhang, H.; Liu, Y.; Xie, R.; He, X.; Zhou, Y.; Liang, L.; Gao, H. The Protein Corona Hampers the Transcytosis of Transferrin-Modified Nanoparticles through Blood-Brain Barrier and Attenuates Their Targeting Ability to Brain Tumor. Biomaterials 2021, 274, 120888.
- 169 Wu, L.; Liu, M.; Shan, W.; Zhu, X.; Li, L.; Zhang, Z.; Huang, Y. Bioinspired Butyrate-Functionalized Nanovehicles for Targeted Oral Delivery of Biomacromolecular Drugs. J. Control. Release 2017, 262, 273–283.
- 170 Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D. S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596.
- 171 Qiao, R.; Jia, Q.; Hüwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H. J.; Gao, M. Receptor-Mediated Delivery of Magnetic Nanoparticles across the Blood-Brain Barrier. ACS Nano 2012, 6, 3304–3310.
- 172 Wang, L.; Jiang, W.; Xiao, L.; Li, H.; Chen, Z.; Liu, Y.; Dou, J.; Li, S.; Wang, Q.; Han, W.; Wang, Y.; Liu, H. Self-Reporting and Splitting Nanopomegranates Potentiate Deep Tissue Cancer Radiotherapy via Elevated Diffusion and Transcytosis. ACS Nano 2020, 14, 8459–8472.
- 173 Sheth, V.; Wang, L.; Bhattacharya, R.; Mukherjee, P.; Wilhelm, S. Strategies for Delivering Nanoparticles across Tumor Blood Vessels. Adv. Funct. Mater. 2020, 31, 2007363.
- 174 Zhang, Z.; Wang, T.; Yang, R.; Fu, S.; Guan, L.; Hou, T.; Mu, W.; Pang, X.; Liang, S.; Liu, Y.; Zhang, N. Small Morph Nanoparticles for Deep Tumor Penetration via Caveolae-Mediated Transcytosis. ACS Appl. Mater. Interfaces 2020, 12, 38499–38511.
- 175 Wang, G.; Zhou, Z.; Zhao, Z.; Li, Q.; Wu, Y.; Yan, S.; Shen, Y.; Huang, P. Enzyme-Triggered Transcytosis of Dendrimer-Drug Conjugate for Deep Penetration into Pancreatic Tumors. ACS Nano 2020, 14, 4890–4904.
- 176 Zhou, Q.; Li, J.; Xiang, J.; Shao, S.; Zhou, Z.; Tang, J.; Shen, Y. Transcytosis-Enabled Active Extravasation of Tumor Nanomedicine. Adv. Drug Deliv. Rev. 2022, 189, 114480.
- 177 Xiang, J.; Shen, Y.; Zhang, Y.; Liu, X.; Zhou, Q.; Zhou, Z.; Tang, J.; Shao, S.; Shen, Y. Multipotent Poly(Tertiary Amine-Oxide) Micelles for Efficient Cancer Drug Delivery. Adv. Sci. 2022, 9, e2200173.
- 178 Chen, S.; Zhong, Y.; Fan, W.; Xiang, J.; Wang, G.; Zhou, Q.; Wang, J.; Geng, Y.; Sun, R.; Zhang, Z.; Piao, Y.; Wang, J.; Zhuo, J.; Cong, H.; Jiang, H.; Ling, J.; Li, Z.; Yang, D.; Yao, X.; Xu, X.; Zhou, Z.; Tang, J.; Shen, Y. Enhanced Tumour Penetration and Prolonged Circulation in Blood of Polyzwitterion-Drug Conjugates with Cell-Membrane Affinity. Nat. Biomed. Eng. 2021, 5, 1019–1037.
- 179 Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; Gan, Z.; Mo, R.; Gu, Z.; Shen, Y. Enzyme-Activatable Polymer-Drug Conjugate Augments Tumour Penetration and Treatment Efficacy. Nat. Nanotechnol. 2019, 14, 799–809.
- 180 Cox, T. R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238.
- 181 Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J. Cell. Biochem. 2019, 120, 2782–2790.




