Efficient Photolytic Halogenation and Oxidation of Unactivated Alkyl sp3 C—H Bonds with Iodine(III)
Hao Jia
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorNan Li
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorChunmei Tang
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorYajuan Wang
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorYonghao Xi
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorRongbao Liao
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorWei Xu
Institute of Marine Biomedicine, Shenzhen Polytechnic, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Fufang Wu
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiaobao Shen
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hongbin Zhai
State Key Laboratory of Chemical Oncogenomics, Shenzhen Engineering Laboratory of Nano Drug Slow-Release, Peking University Shenzhen Graduate School, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorHao Jia
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorNan Li
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorChunmei Tang
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorYajuan Wang
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorYonghao Xi
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorRongbao Liao
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
Search for more papers by this authorWei Xu
Institute of Marine Biomedicine, Shenzhen Polytechnic, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Fufang Wu
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xiaobao Shen
Biomass Oligosaccharides Engineering Technology Research Center of Anhui Province, Engineering Research Center of Biomass Conversion and Pollution Prevention of Anhui Educational Institutions, Fuyang Normal University, Fuyang, Anhui, 236037 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Hongbin Zhai
State Key Laboratory of Chemical Oncogenomics, Shenzhen Engineering Laboratory of Nano Drug Slow-Release, Peking University Shenzhen Graduate School, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
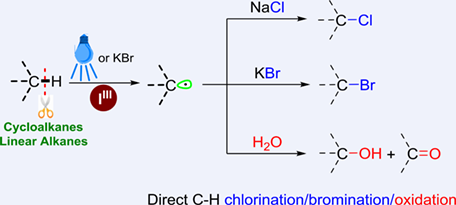
A metal-free, green, and sustainable functionalization of unactivated alkyl sp3 C—H bonds is reported using iodine(III) as a feasible dehydrogenation agent under visible light or KBr, and alkyl chlorides, bromides, alcohols, and ketones could be constructed by addition of different coupling reagents. Cheap and safe iodobenzene diacetate was used to form a radical to activate the alkyl sp3 C—H bond in a highly efficient manner, which can construct different alkylation products by adding corresponding coupling reagents.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300544-sup-0001-supinfo.pdfPDF document, 6.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Kamata, K.; Yonehara, K.; Nakagawa, Y.; Uehara, K.; Mizuno, N. Efficient stereo- and regioselective hydroxylation of alkanes catalysed by a bulky polyoxometalate. Nat. Chem. 2010, 2, 478–483; (b) Lv, Y.; Li, Y.; Xiong, T.; Lu, Y.; Liu, Q.; Zhang, Q. nBu4NI-catalyzed oxidative imidation of ketones with imides: synthesis of α-amino ketones. Chem. Commun. 2014, 50, 2367–2369; (c) Xu, C.; Li, X.; Bai, L. Direct Aerobic α-Hydroxylation of Arylacetates for the Synthesis of Mandelates. J. Org. Chem. 2022, 87, 4298–4304; (d) Huang, L.; Bismuto, A.; Rath, S. A.; Trapp, N.; Morandi, B. Ruthenium-Catalyzed Dehydrogenation Through an Intermolecular Hydrogen Atom Transfer Mechanism. Angew. Chem. Int. Ed. 2021, 60, 7290–7296.
- 2 Wang, Q.; Ni, S.; Wang, X.; Wang, Y.; Pan, Y. Visible-light-mediated tungsten-catalyzed C–H amination of unactivated alkanes with nitroarenes. Sci. China Chem. 2022, 65, 678–685.
- 3(a) Hu, A.; Guo, J.; Pan, H.; Zuo, Z. Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science. 2018, 361, 668–672; (b) Wang, Z.; Wang, F. Radical-Mediated Selective Functionalization of Unactivated Primary C–H Bonds. Chin. J. Chem. 2022, 40, 1751–1753; (c) Chan, H.; Yang, J.; Yu, J. Catalyst-controlled site-selective methylene C–H lactonization of dicarboxylic acids. Science 2022, 376, 1481–1487.
- 4(a) Liu, S.; Zhang, Q.; Tian, X.; Fan, S.; Huang, J.; Whiting, A. Highly selective halogenation of unactivated C(sp3)–H with NaX under co-catalysis of visible light and Ag/AgX. Green Chem. 2018, 20, 4729–4737; (b) Manabe, Y.; Kitawaki, Y.; Nagasaki, M.; Fukase, K.; Matsubara, H.; Hino, Y.; Fukuyama, T.; Ryu, I. Revisiting the bromination of C−H bonds with molecular bromine by using a photo-microflow system. Chem. Eur. J. 2014, 20, 12750–12753.
- 5(a) Lee, B. J.; DeGlopper, K. S.; Yoon, T. P. Site-Selective Alkoxylation of Benzylic C−H Bonds by Photoredox Catalysis. Angew. Chem. Int. Ed. 2020, 59, 197–202; (b) Li, S.; Su, M.; Sun, J.; Hu, K.; Jin, J. Visible Light-Promoted Magnesium, Iron, and Nickel Catalysis Enabling C(sp3)–H Lactonization of 2-Alkylbenzoic Acids. Org. Lett. 2021, 23, 5842–5847; (c) Yamane, D.; Tanaka, H.; Hirata, A.; Tamura, Y.; Takahashi, D.; Takahashi, Y.; Nagamitsu, T.; Ohtawa, M. One-Pot gamma-Lactonization of Homopropargyl Alcohols via Intramolecular Ketene Trapping. Org. Lett. 2021, 23, 2831–2835.
- 6(a) Li, Z.; Luo, L.; Li, M.; Chen, W.; Liu, Y.; Yang, J.; Xu, S. M.; Zhou, H.; Ma, L.; Xu, M.; Kong, X.; Duan, H. Photoelectrocatalytic C–H halogenation over an oxygen vacancy-rich TiO2 photoanode. Nat. Commun. 2021, 12, 6698; (b) Zhang, Q.; Liu, S.; Tian, X.; Liu, Y.; Fan, S.; Huang, B.; Whiting, A. Cu@CuCl-visible light co-catalysed chlorination of C(sp3)–H bonds with MCln solution and photocatalytic serial reactor-based synthesis of benzyl chloride. Green Chem. 2022, 24, 384–393; (c) Tateno, H.; Iguchi, S.; Miseki, Y.; Sayama, K. Photo-Electrochemical C–H Bond Activation of Cyclohexane Using a WO3 Photoanode and Visible Light. Angew. Chem. Int. Ed. 2018, 57, 11238–11241; (d) Coutard, N.; Goldberg, J. M.; Valle, H. U.; Cao, Y.; Jia, X.; Jeffrey, P. D.; Gunnoe, T. B.; Groves, J. T. Aerobic Partial Oxidation of Alkanes Using Photodriven Iron Catalysis. Inorg. Chem. 2022, 61, 759–766; (e) Maeda, B.; Sakakibara, Y.; Murakami, K.; Itami, K. Photoredox-Catalyzed Benzylic Esterification via Radical-Polar Crossover. Org. Lett. 2021, 5113–5117; (f) Jacoby, C.; Ferlaino, S.; Bezold, D.; Jessen, H.; Muller, M.; Boll, M. ATP-dependent hydroxylation of an unactivated primary carbon with water. Nat. Commun. 2020, 11, 3906; (g) Qiu, Y.; Hartwig, J. F. Mechanism of Ni-Catalyzed Oxidations of Unactivated C(sp3)–H Bonds. J. Am. Chem. Soc. 2020, 142, 19239–19248; (h) Kawamata, Y.; Yan, M.; Liu, Z.; Bao, D. H.; Chen, J.; Starr, J. T.; Baran, P. S. Scalable, Electrochemical Oxidation of Unactivated C–H Bonds. J. Am. Chem. Soc. 2017, 139, 7448–7451; (i) Bigi, M. A.; Reed, S. A.; White, M. C. Diverting non-haem iron catalysed aliphatic C–H hydroxylations towards desaturations. Nat. Chem. 2011, 3, 216–222; (j) Quinn, R. K.; Konst, Z. A.; Michalak, S. E.; Schmidt, Y.; Szklarski, A. R.; Flores, A. R.; Nam, S.; Horne, D. A.; Vanderwal, C. D.; Alexanian, E. J. Site-Selective Aliphatic C–H Chlorination Using N-Chloroamides Enables a Synthesis of Chlorolissoclimide. J. Am. Chem. Soc. 2016, 138, 696–702; (k) Zhu, X.; Liu, Y.; Liu, C.; Yang, H.; Fu, H. Light and oxygen-enabled sodium trifluoromethanesulfinate-mediated selective oxidation of C–H bonds. Green Chem. 2020, 22, 4357–4363; (l) Garcia-Bosch, I.; Siegler, M. A. Copper-Catalyzed Oxidation of Alkanes with H2O2 under a Fenton-like Regime. Angew. Chem. Int. Ed. 2016, 55, 12873–12876; (m) Li, J.; Liu, J. Fe (III)-Catalyzed Aerobic Oxidation of 1,4-Diols. Chin. J. Chem. 2023, 41, 1963–1966.
- 7(a) Zhao, M.; Lu, W. Visible Light-Induced Oxidative Chlorination of Alkyl sp3 C–H Bonds with NaCl/Oxone at Room Temperature. Org. Lett. 2017, 19, 4560–4563; (b) Kiyokawa, K.; Ito, R.; Takemoto, K.; Minakata, S. C–H oxygenation at tertiary carbon centers using iodine oxidant. Chem. Commun. 2018, 54, 7609–7612.
- 8(a) Zhao, M.; Lu, W. Catalytic Bromination of Alkyl sp3 C–H Bonds with KBr/Air under Visible Light. Org. Lett. 2018, 20, 5264–5267; (b) Rana, S.; Biswas, J. P.; Sen, A.; Clemancey, M.; Blondin, G.; Latour, J. M.; Rajaraman, G.; Maiti, D. Selective C−H halogenation over hydroxylation by non-heme iron(iv)-oxo. Chem. Sci. 2018, 9, 7843–7858.
- 9 Richers, J.; Heilmann, M.; Drees, M.; Tiefenbacher, K. Synthesis of Lactones via C–H Functionalization of Nonactivated C(sp3)–H Bonds. Org. Lett. 2016, 18, 6472–6475.
- 10 Tanaka, K. III; Yoshida, M.; Yamamoto, A.; Hashimoto, Y.; Morita, N.; Tamura, O. Synthesis of N-Aryl Isoxazolidines by Photo-Promoted N-Selective Arylation of Oximes and Cyclization Using Hypervalent Iodine Reagents and Copper Catalyst. Adv. Synth. Catal. 2023, 365, 1419–1424.
- 11(a) Moteki, S. A.; Usui, A.; Zhang, T.; Solorio Alvarado, C. R.; Maruoka, K. Site-selective oxidation of unactivated C(sp3)–H bonds with hypervalent iodine(III) reagents. Angew. Chem. Int. Ed. 2013, 52, 8657–8660; (b) Moteki, S. A.; Selvakumar, S.; Zhang, T.; Usui, A.; Maruoka, K. A Practical Approach for the Oxidation of Unactivated C(sp3)–H Bonds with o-Nitro(diacetoxyiodo)benzene as an Efficient Hypervalent Iodine(III)-Based Oxidizing Agent. Asian J. Org. Chem. 2014, 3, 932–935; (c) Matsumoto, A.; Lee, H. J.; Maruoka, K. Development of New Radical-mediated Selective Reactions Promoted by Hypervalent Iodine(III) Reagents. Chem. Rec. 2021, 21, 1342–1357.
- 12 Zhao, Y.; Yim, W.-L.; Tan, C. K.; Yeung, Y.-Y. An Unexpected Oxidation of Unactivated Methylene C–H Using DIB/TBHP Protocol. Org. Lett. 2011, 13, 4308–4311.
- 13 Wu, F.; Han, X.; Li, X.; Shen, X.; Wang, C.; Tian, Z.; Cheng, B.; Zhang, J.; Sheng, L.; Zhai, H. Iodine(III) promotes cross-dehydrogenative coupling of N-hydroxyphthalimide and unactivated C(sp3)–H bonds. Commun. Chem. 2021, 4, 46.
- 14 Sakamoto, R.; Inada, T.; Selvakumar, S.; Moteki, S. A.; Maruoka, K. Efficient photolytic C–H bond functionalization of alkylbenzene with hypervalent iodine(III) reagent. Chem. Commun. 2016, 52, 3758–3761.
- 15 Dohi, T.; Takenaga, N.; Goto, A.; Maruyama, A.; Kita, Y. Direct Lactone Formation by Using Hypervalent lodine(II) Reagents with KBr via Selective C–H Abstraction Protocol. Org. Lett. 2007, 9, 3129–3132.




