Electrochemical Reduction of Benzo[b]thiophene 1,1-Dioxides with HFIP as Hydrogen Donor†
Ming-Zhong Guo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorMei-Jin Mou
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorZhuo Chen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorShao-Fei Ni
Department of Chemistry and Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorMing Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorCorresponding Author
Li-Rong Wen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Lin-Bao Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this authorMing-Zhong Guo
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorMei-Jin Mou
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorZhuo Chen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorShao-Fei Ni
Department of Chemistry and Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, Shantou, Guangdong, 515063 China
Search for more papers by this authorMing Li
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
Search for more papers by this authorCorresponding Author
Li-Rong Wen
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Lin-Bao Zhang
State Key Laboratory Base of Eco-Chemical Engineering, College of Chemistry and Molecular Engineering, Qingdao University of Science & Technology, Qingdao, Shandong, 266042 China
E-mail: [email protected], [email protected]Search for more papers by this author† Dedicated to the Special Issue of Electrocatalysis.
Comprehensive Summary
A straightforward electrochemical reduction of benzo[b]thiophene 1,1-dioxides with HFIP as the hydrogen donor has been reported in an undivided cell under metal-free conditions. Moreover, the tolerance of various functional groups and scaled-up experiments showed the practicability and potential applications of this methodology.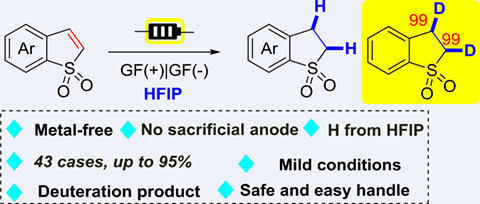
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300527-sup-0001-Supinfo.pdfPDF document, 6.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Wendelin, F.; Heiner, G.; Stefan, T.; Ralf, E. Novel aromatic glycoside derivatives, medicaments containing said compounds, and the use thereof. WO2011107494, 2011; (b) Dixon, D.; Grina, J.; Josey, J. A.; Rizzi, J. P.; Schlachter, S. T.; Wallace, E. M.; Wang, B.; When, P.; Xu, R.; Yang, H. Cyclic sulfone and sulfoximine analogs and uses thereof. WO2015095048, 2015; (c) When, P.; Yang, P. Tricyclic inhibitors of HIF-2-alpha and uses thereof. US20160362390, 2016; (d) Aigars, L.; Gundars, L.; Ivars, K.; Daniel, B.; Paul, F.; Nagma, K. Bicyclosulfonyl acid (BCSA) compounds and their use as therapeuticagents. WO2008142376, 2008.
- 2(a) Glasebrook, A. L.; Misner, J. W.; Stephenson, G. A.; Schmid, C. R. Synthesis and biological activity of trans-2,3-dihydroraloxifene. Bioorg. Med. Chem. Lett. 1999, 9, 1137–1140; (b) Krajewski, K.; Zhang, Y. J.; Parrish, D.; Deschamps, J.; Roller, P.; Pathak, V. K. New HIV-1 reverse transcriptase inhibitors based on a tricyclic benzothiophene scaffold: Synthesis, resolution, and inhibitory activity. Bioorg. Med. Chem. Lett. 2006, 16, 3034–3038.
- 3(a) Wang, D.-S.; Chen, Q.-A.; Li, W.; Yu, C.-B.; Zhou, Y.-G.; Zhang, X. Pd-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles Activated by Brønsted Acids. J. Am. Chem. Soc. 2010, 132, 8909–8911; (b) Wang, D.-S.; Tang, J.; Zhou, Y.-G.; Chen, M.-W.; Yu, C.-B.; Duan, Y.; Jiang, G.-F. Dehydration triggered asymmetric hydrogenation of 3-(α-hydroxyalkyl)indoles. Chem. Sci. 2011, 2, 803–806; (c) Duan, Y.; Li, L.; Chen, M.-W.; Yu, C.-B.; Fan, H.-J.; Zhou, Y.-G. Homogenous Pd-Catalyzed Asymmetric Hydrogenation of Unprotected Indoles: Scope and Mechanistic Studies. J. Am. Chem. Soc. 2014, 136, 7688–7700; (d) Touge, T.; Arai, T. Asymmetric Hydrogenation of Unprotected Indoles Catalyzed by η6-Arene/N-Me-sulfonyldiamine-Ru(II) Complexes. J. Am. Chem. Soc. 2016, 138, 11299–11305; (e) Yang, Z.; Chen, F.; He, Y.; Yang, N.; Fan, Q.-H. Highly Enantioselective Synthesis of Indolines: Asymmetric Hydrogenation at Ambient Temperature and Pressure with Cationic Ruthenium Diamine Catalysts. Angew. Chem. Int. Ed. 2016, 55, 13863–13866; (f) Ortega, N.; Urban, S.; Beiring, B.; Glorius, F. Ruthenium NHC Catalyzed Highly Asymmetric Hydrogenation of Benzofurans. Angew. Chem. Int. Ed. 2012, 51, 1710–1713; (g) Pauli, L.; Tannert, R.; Scheil, R.; Pfaltz, A. Asymmetric Hydrogenation of Furans and Benzofurans with Iridium–Pyridine–Phosphinite Catalysts. Chem. Eur. J. 2015, 21, 1482–1487.
- 4(a) Bordwell, F. G.; McKellin, W. H. Benzothiophene1 Chemistry. IV.2 Some Addition Reactions of Benzothiophene-1-dioxide3. J. Am. Chem. Soc. 1950, 72, 1985–1988;
(b) Madec, D.; Mingoia, F.; Macovei, C.; Maitro, G.; Giambastiani, G.; Poli, G. Eur. J. Org. Chem. 2005, 3, 552–557;
10.1002/ejoc.200400547 Google Scholar(c) Sakairi, M.; Kogami, M.; Torii, M.; Kataoka, H.; Fujieda, H.; Makino, M.; Kataoka, D.; Okamoto, R.; Miyazawa, T.; Okabe, M.; Inoue, M.; Takahashi, N.; Harada, S.; Watanabe, N. Synthesis and SAR studies of bicyclic amine series GPR119 agonists. Bioorg. Med. Chem. Lett. 2012, 22, 5123–5128; (d) Jeanguenat, A.; Durieux, P.; Edmunds, A. J. F.; Hall, R. G.; Hughes, D.; Loiseleur, O.; Pabba, J.; Stoller, A.; Trah, S.; Wenger, J.; Dutton, A.; Crossthwaite, A. Bicyclic heterocyclic anthranilic diamides as ryanodine receptor modulators with insecticidal activity. Bioorg. Med. Chem. 2016, 24, 403–427; (e) Wu, S. W.; Xu, C.; Xia, K. J.; Lin, Y.; Tian, S.; Ma, H. K.; Ji, Y. T.; Zhu, F.; He, S. D.; Zhang, X. H. Ring closure strategy leads to potent RIPK3 inhibitors. Eur. J. Med. Chem. 2021, 217, 113327; (f) Bordwell, F. G.; Stange, H. Benzothiophene Chemistry. VII. Substitution Reactions of 5-Hydroxy- and 5-Aminobenzothiophene Derivatives. J. Am. Chem. Soc. 1955, 77, 5939–5944; (g) Baumgarth, M.; Beier, N.; Gericke, R. Bicyclic Acylguanidine Na+/H+ Antiporter Inhibitors. J. Med. Chem. 1998, 41, 3736–3747; (h) Ivie, J. A.; Bamberger, N. D.; Parida, K. N.; Shepard, S.; Dyer, D.; Saraiva-Souza, A.; Himmelhuber, R.; McGrath, D. V.; Smeu, M.; Monti, O. L. A. Correlated Energy-Level Alignment Effects Determine Substituent-Tuned Single-Molecule Conductance. ACS Appl. Mater. Interfaces 2021, 13, 4267–4277.
- 5(a) Kingston, C.; Palkowitz, M. D.; Takahira, Y.; Vantourout, J. C.; Peters, B. K.; Kawamata, Y.; Baran, P. S. A Survival Guide for the “Electro-curious”. Acc. Chem. Res. 2020, 53, 72–83;
(b) Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 2021, 50, 7941–8002;
(c) Huang, B.; Sun, Z.; Sun, G. Recent progress in cathodic reduction-enabled organic electrosynthesis: Trends, challenges, and opportunities. eScience 2022, 2, 243–277;
10.1016/j.esci.2022.04.006 Google Scholar(d) Malapit, C. A.; Prater, M. B.; Cabrera-Pardo, J. R.; Li, M.; Pham, T. D.; McFadden, T. P.; Blank, S.; Minteer, S. D. Advances on the Merger of Electrochemistry and Transition Metal Catalysis for Organic Synthesis. Chem. Rev. 2022, 122, 3180–3218; (e) Ackermann, L. Metalla-electrocatalyzed C–H Activation by Earth-Abundant 3d Metals and Beyond. Acc. Chem. Res. 2020, 53, 84–104; (f) Yuan, Y.; Yang, J.; Lei, A. Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals. Chem. Soc. Rev. 2021, 50, 10058–10086; (g) Xiong, P.; Xu, H.-C. Chemistry with Electrochemically Generated N-Centered Radicals. Acc. Chem. Res. 2019, 52, 3339–3350; (h) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319; (i) Tan, Z.-M.; Zhang, H.-N.; Xu, K.; Zeng, C.-C. Electrochemical Radical-Polar Crossover Since 2020: A Radical Approach to Polar Chemistry. 2023, Sci. Chin. Chem. 66, 10.1007/s11426-023-1735-x.
- 6(a) Hioki, Y.; Costantini, M.; Griffin, J.; Harper, K. C.; Merini, M. P.; Nissl, B.; Kawamata, Y.; Baran, P. S. Overcoming the limitations of Kolbe coupling with waveform-controlled electrosynthesis. Science 2023, 380, 81–87; (b) Münchow, T. V.; Dana, S.; Xu, Y.; Yuan, B.; Ackermann, L. Enantioselective electrochemical cobalt-catalyzed aryl C–H activation reactions. Science 2023, 379, 1036–1042; (c) Sun, G. Q.; Yu, P.; Zhang, W.; Zhang, W.; Wang, Y.; Liao, L. L.; Zhang, Z.; Li, L.; Lu, Z.; Yu, D. G.; Lin, S. Electrochemical reactor dictates site selectivity in N-heteroarene carboxylations. Nature 2023, 615, 67–72; (d) Gao, Y.; Zhang, B.; He, J.; Baran, P. S. Ni-Electrocatalytic Enantioselective Doubly Decarboxylative C(sp3)–C(sp3) Cross Coupling. J. Am. Chem. Soc. 2023, 145, 11518–11523; (e) Yang, D. F.; Guan, Z. P.; Peng, Y. A.; Zhu, S. X.; Wang, P. J.; Huang, Z. L.; Alhumade, H.; Gu, D.; Yi, H.; Lei, A. Electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles. Nat. Commun. 2023, 14, 1476–1484; (f) Liao, L.-L.; Wang, Z.-H.; Cao, K.-G.; Sun, G.-Q.; Zhang, W.; Ran, C.-K.; Li, Y.; Chen, L.; Cao, G.-M.; Yu, D.-G. Electrochemical Ring-Opening Dicarboxylation of Strained Carbon–Carbon Single Bonds with CO2: Facile Synthesis of Diacids and Derivatization into Polyesters. J. Am. Chem. Soc. 2022, 144, 2062–2068; (g) Huang, H.; Steiniger, K. A.; Lambert, T. H. Electrophotocatalysis: Combining Light and Electricity to Catalyze Reactions. J. Am. Chem. Soc. 2022, 144, 12567–12583; (h) Hamby, T. B.; LaLama, M. J.; Sevov, C. S. Controlling Ni redox states by dynamic ligand exchange for electroreductive Csp3–Csp2 coupling. Science 2022, 376, 410–416; (i) Jiao, K.-J.; Liu, D.; Ma, H.-X.; Qiu, H.; Fang, P.; Mei, T.-S. Nickel-Catalyzed Electrochemical Reductive Relay Cross-Coupling of Alkyl Halides to Aryl Halides. Angew. Chem. Int. Ed. 2020, 59, 6520–6524; (j) Yin, Z.-C.; Niu, C.; Li, M. J.; Liu, W.-R.; Wang, G.-W. Regioselective Electrochemical Hydroalkylations of [60]Fullerene-Fused Furochromenone. Chin. J. Chem. 2022, 41, 769–775; (k) Lian, F.; Luo, F. X.; Wang, M.; Xu, K.; Zeng, C. C. Electrochemical Atom Transfer Radical Addition of Polychloroalkanes to Olefins Promoted by 4,4-Di-tert-butyl Bipyridine. Chin. J. Chem. 2022, 41, 1583–1588; (l) Li, Z.-M.; Shuai, B.; Ma, C.; Fang, P.; Mei, T.-S. Nickel-Catalyzed Electroreductive Syntheses of Triphenylenes Using ortho-Dihalobenzene-Derived Benzynes. Chin. J. Chem. 2022, 40, 2335–2344; (m) Zeng, L.; Qin, J.-H.; Lv, G.-F.; Hu, M.; Sun, Q.; Ouyang, X.-H.; He, D.-L.; Li, J.-H. Electrophotocatalytic Reductive 1,2-Diarylation of Alkenes with Aryl Halides and Cyanoaromatics. Chin. J. Chem. 2023, 41, 1921–1930; (n) Liang, Y. W.; Niu, L. B.; Liang, X.-A.; Wang, S. C.; Wang, P. J.; Lei, A. W. Electrooxidation-Induced C(sp3)–H/C(sp2)–H Radical-Radical Cross-Coupling between Xanthanes and Electron-Rich Arenes. Chin. J. Chem. 2022, 40, 1422–1428; (o) Li, Y, L.; Wang, H. M.; Zhang, H.; Lei, A. W. Electrochemical Dimethyl Sulfide-Mediated Esterification of Amino Acids. Chin. J. Chem. 2021, 39, 3023–3028.
- 7(a) Li, J.; He, L.; Liu, X.; Cheng, X.; Li, G. Electrochemical Hydrogenation with Gaseous Ammonia. Angew. Chem. Int. Ed. 2019, 58, 1759–1763; (b) Liu, X.; Liu, R.; Qiu, J.; Cheng, X.; Li, G. Chemical-Reductant-Free Electrochemical Deuteration Reaction using Deuterium Oxide. Angew. Chem. Int. Ed. 2020, 59, 13962–13967; (c) Qin, Y.; Lu, J.; Zou, Z.; Hong, H.; Li, Y.; Li, Y.; Chen, L.; Hu, J.; Huang, Y. Metal-free chemoselective hydrogenation of unsaturated carbon–carbon bonds via cathodic reduction. Org. Chem. Front. 2020, 7, 1817–1822; (d) Huang, B. B.; Li, Y.; Yang, C.; Xia, W. J. Electrochemical 1,4-reduction of α,β-unsaturated ketones with methanol and ammonium chloride as hydrogen sources. Chem. Commun. 2019, 55, 6731–6734; (e) Qin, H. Y.; Yang, J. J.; Yan, K. L.; Xue, Y. X.; Zhang, M. C.; Sun, X. J.; Wen, J. W.; Wang, H. Electrochemical-Induced Hydrogenation of Electron-Deficient Internal Olefins and Alkynes with CH3OH as Hydrogen Donor. Adv. Synth. Catal. 2021, 363, 2104–2109.
- 8(a) Wen, J.; Qin, H.; Yan, K.; Yang, X.; Sun, X.; Wei, W.; Yang, J.; Wang, H. Electrochemical-Induced Transfer Hydrogenation of Imidazopyridines with Secondary Amine as Hydrogen Donor. Org. Lett. 2020, 22, 8824–8828; (b) Li, M.; Liu, C.; Huang, Y.; Han, S.; Zhang, B. Water-involving transfer hydrogenation and dehydrogenation of N-heterocycles over a bifunctional MoNi4 electrode. Chin. J. Catal. 2021, 42, 1983–1991; (c) Miller, L. L.; Christensen, L. Electrocatalytic hydrogenation of aromatic compounds. J. Org. Chem. 1978, 43, 2059; (d) Peters, B. K.; Rodriguez, K. X.; Reisberg, S. H.; Beil, S. B.; Hickey, D. P.; Kawamata, Y.; Collins, M.; Starr, J.; Chen, L.; Udyavara, S.; Klunder, K.; Gorey, T. J.; Anderson, S. L.; Neurock, M.; Minteer, S. D.; Baran, P. S. Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry. Science 2019, 363, 838–845.
- 9(a) Birch, A. J. Electrolytic Reduction in Liquid Ammonia. Nature 1946, 158, 60; (b) Swenson, K. E.; Zemach, D.; Nanjundiah, C.; Kariv-Miller, E. Birch reductions of methoxyaromatics in aqueous solution. J. Org. Chem. 1983, 48, 1777–1779; (c) Bordeau, M.; Biran, C.; Pons, P.; Leger-Lambert, M. P.; Dunogues, J. The electrochemical reductive trimethylsilylation of aryl chlorides: a good route to aryltrimethylsilanes and a novel route to tris(trimethylsilyl)cyclohexadienes. J. Org. Chem. 1992, 57, 4705–4711; (d) Ishifune, M.; Yamashita, H.; Kera, Y.; Yamashita, N.; Hirata, K.; Murase, H.; Kashimura, S. Electroreduction of aromatics using magnesium electrodes in aprotic solvents containing alcoholic proton donors. Electrochim. Acta 2003, 48, 2405–2409.
- 10(a) Li, Y.; Wen, L.-R.; Guo, W.-S. A guide to organic electroreduction using sacrificial anodes. Chem. Soc. Rev. 2023, 52, 1168–1188; (b) Du, W.-B.; Wang, N.-N.; Pan, C.; Ni, S.-F.; Wen, L.-R.; Li, M.; Zhang, L.-B. Regio- and stereoselective electrochemical synthesis of sulfonylated enethers from alkynes and sulfonyl hydrazides. Green Chem. 2021, 23, 2420–2426; (c) Wang, Z.-C.; Li, R.-T.; Ma, Q.; Chen, J.-Y.; Ni, S.-F.; Li, M.; Wen, L.-R.; Zhang, L.-B. Electrochemically enabled rhodium-catalyzed [4 + 2] annulations of arenes with alkynes. Green Chem. 2021, 23, 9515–9522; (d) Li, R.-T.; Yuan, D.-F.; Ping, M.-Q.; Zhu, Y.-Y.; Ni, S.-F.; Li, M.; Wen, L.-R.; Zhang, L.-B. Electrochemically-promoted synthesis of benzo[b]thiophene-1,1-dioxides via strained quaternary spirocyclization. Chem. Sci. 2022, 13, 9940–9946; (e) Fu, Z.-H.; Tian, H.-D.; Ni, S.-F.; Wright, J. S.; Li, M.; Wen, L.-R.; Zhang, L.-B. Scalable selective electrochemical oxidation of sulfides to sulfoxides. Green Chem. 2022, 24, 4772–4777; (f) Zhang, L.-B.; Geng, R.-S.; Wang, Z.-C.; Ren, G.-Y.; Wen, L.-R.; Li, M. Electrochemical intramolecular C–H/N–H functionalization for the synthesis of isoxazolidine-fused isoquinolin-1(2H)-ones. Green Chem. 2020, 22, 16–21; (g) Ma, Q.; Li, M.; Chen, Z.; Ni, S.-F.; Wright, J. S.; Wen, L.-R.; Zhang, L.-B. An approach for the synthesis of 2-aryl-3-sulfonyl substituted quinolines through an electrochemical cascade annulation pathway. Green Chem. 2022, 24, 4425–4431; (h) Ping, M.-Q.; Guo, M.-Z.; Li, R.-T.; Wang, Z.-C.; Ma, C.; Wen, L.-R.; Ni, S.-F.; Guo, W.-S.; Li, M.; Zhang, L.-B. Electrochemically Promoted [3 + 2] Annulation of Imidazo[1,2-a]pyridine with Alkynes. Org. Lett. 2022, 24, 7410–7415; (i) Tian, H.-D.; Fu, Z.-H.; Li, C.; Lin, H.-C.; Li, M.; Ni, S.-F.; Wen, L.-R.; Zhang, L.-B. Selective Electrochemical Synthesis of 9-Aryl-10-sulfonyl Substituted Phenanthrene from Alkynes and Sulfonyl Hydrazides. Org. Lett. 2022, 24, 9322–9326; (j) Wen, L.-R.; Wang, N.-N.; Du, W.-B.; Zhu, M.-Z.; Pan, C.; Zhang, L.-B.; Li, M. Electrochemical Selective Oxidative Synthesis of Diversified Sulfur Heterocycles from β-Ketothioamides. Chin. J. Chem. 2021, 39, 1831–1837.
- 11(a) Zhang, X.; Yang, C.; Gao, H.; Wang, L.; Guo, L.; Xia, W. J. Reductive Arylation of Aliphatic and Aromatic Aldehydes with Cyanoarenes by Electrolysis for the Synthesis of Alcohols. Org. Lett. 2021, 23, 3472–3476; (b) Li, H.; Breen, C.; Seo, H.; Jamison, T.; Fang, Y.-Q.; Bio, M. Ni-Catalyzed Electrochemical Decarboxylative C-C Couplings in Batch and Continuous Flow. Org. Lett. 2018, 20, 1338–1341.
- 12(a) Niu, C.; Yang, J. J.; Yan, K. L.; Xie, J. F.; Jiang, W.; Li, B. W.; Wen, J. W. Electrochemical ammonium-cation-assisted pyridylation of inert N-heterocycles via dual-proton-coupled electron transfer. iScience 2022, 25, 104253; (b) Yang, J. J.; Ma, J.; Yan, K. L.; Tian, L. J.; Li, B. W.; Wen, J. W. Electrochemical Ammonium Cation-Assisted Hydropyridylation of Ketone-Activated Alkenes: Experimental and Computational Mechanistic Studies. Adv. Synth. Catal. 2022, 364, 845–854.




