Rhodium-Catalyzed Asymmetric Transfer Hydrogenation of Heterocyclic Diaryl Ketones: Facile Access to Key Intermediate of Baloxavir†
Li Wang
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorRenwei Xiao
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorJingyuan Song
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorLong-Sheng Zheng
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorCorresponding Author
Qiwei Lang
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Gen-Qiang Chen
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xumu Zhang
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, Guangdong, 515031 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorLi Wang
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorRenwei Xiao
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorJingyuan Song
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorLong-Sheng Zheng
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Search for more papers by this authorCorresponding Author
Qiwei Lang
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Gen-Qiang Chen
Academy for Advanced Interdisciplinary Studies, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Xumu Zhang
Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen, Guangdong, 518000 China
Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, Guangdong, 515031 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
Transition metal-catalyzed asymmetric transfer hydrogenation has been proven to be a powerful approach for the synthesis of chiral alcohols. Herein, a highly efficient and enantioselective transfer hydrogenation of dibenzoheptaheterocyclic ketones catalyzed by an arene-tethered TsDPEN-based Rh(III) catalyst has been successfully developed, and a variety of dibenzoheptaheterocyclic ketones were reduced by a 1/1 mixture of formic acid and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) with high yields and enantioselectivities. With this method, the asymmetric reduction of 7,8-difluorodibenzo[b,e]thiepin-11(6H)-one has been realized, providing the key intermediate of baloxavir marboxil with >99% yield and >99% ee at a substrate/catalyst molar ratio of 1000.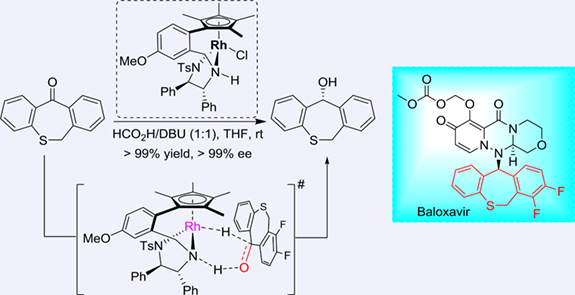
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300487-sup-0001-supinfo.pdfPDF document, 5.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Heo, Y.-A. Baloxavir: First Global Approval. Drugs 2018, 78, 693–697.
- 2 Hayden, F. G.; Sugaya, N.; Hirotsu, N.; Lee, N.; de Jong, M. D.; Hurt, A. C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; Kawaguchi, K.; Shishido, T.; Arai, M.; Tsuchiya, K.; Uehara, T.; Watanabe, A.; Baloxavir Marboxil Investigators, G. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N. Engl. J. Med. 2018, 379, 913–923.
- 3 Shibahara, S.; Fukui, N.; Maki, T. Polycyclic pyridone derivative, crystal and preparation method thereof. JP6212678, 2017.
- 4 Zheng, X.; Zhang, Y.; Fu, C.; Wu, Y. Synthetic method of anti-influenza drug baloflavone Baloxavir marboxil. CN109504721, 2019.
- 5 Zhang, F.; Jiang, L.; Qiu, Y.; Ni, G.; Wang, H.; Liu, N.; Chen, Y.; Jiang, J. Preparation method of chiral dibenzo[b,e]thiepin-11-ol for preparation of baloxavir marboxil as antiviral drug. CN110143944, 2019.
- 6 Zi, C.; Zeng, L.; Wang, Y. Method for synthesizing baloxavir marboxil intermediate from 7,8-difluorodibenzo[b,e]thiepin-11(6H)-one. CN112812095, 2021.
- 7(a) Yin, C.; Jiang, Y. F.; Huang, F.; Xu, C. Q.; Pan, Y.; Gao, S.; Chen, G. Q.; Ding, X.; Bai, S. T.; Lang, Q.; Li, J.; Zhang, X. A 13-million turnover-number anionic Ir-catalyst for a selective industrial route to chiral nicotine. Nat. Commun. 2023, 14, 3718;
(b) Yu, J.; Huang, F.; Fang, W.; Yin, C.; Shi, C.; Lang, Q.; Chen, G.-Q.; Zhang, X. Discovery and development of ferrocene-based tetradentate ligands for Ir-catalysed asymmetric hydrogenation of ketone. Green Synth. Catal. 2022, 3, 175–178;
10.1016/j.gresc.2022.03.004 Google Scholar(c) Zhao, Q.; Chen, C.; Wen, J.; Dong, X.-Q.; Zhang, X. Noncovalent Interaction-Assisted Ferrocenyl Phosphine Ligands in Asymmetric Catalysis. Acc. Chem. Res. 2020, 53, 1905–1921; (d) Liang, Z.; Yang, T.; Gu, G.; Dang, L.; Zhang, X. Scope and Mechanism on Iridium-f-Amphamide Catalyzed Asymmetric Hydrogenation of Ketones. Chin. J. Chem. 2018, 36, 851–856; (e) Yu, J.; Duan, M.; Wu, W.; Qi, X.; Xue, P.; Lan, Y.; Dong, X.-Q.; Zhang, X. Readily Accessible and Highly Efficient Ferrocene-Based Amino-Phosphine-Alcohol (f-Amphol) Ligands for Iridium-Catalyzed Asymmetric Hydrogenation of Simple Ketones. Chem. Eur. J. 2017, 23, 970–975; (f) Wu, W.; Liu, S.; Duan, M.; Tan, X.; Chen, C.; Xie, Y.; Lan, Y.; Dong, X.-Q.; Zhang, X. Iridium Catalysts with f-Amphox Ligands: Asymmetric Hydrogenation of Simple Ketones. Org. Lett. 2016, 18, 2938–2941; (g) Liu, D.; Gao, W.; Wang, C.; Zhang, X. Practical synthesis of enantiopure γ-amino alcohols by rhodium-catalyzed asymmetric hydrogenation of β-secondary-amino ketones. Angew. Chem. Int. Ed. 2005, 44, 1687–1689; (h) Tang, W.; Zhang, X. A chiral 1,2-bisphospholane ligand with a novel structural motif: applications in highly enantioselective Rh-catalyzed hydrogenations. Angew. Chem. Int. Ed. 2002, 41, 1612–1614.10.1002/1521-3773(20020503)41:9<1612::AID-ANIE1612>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 8(a) Jiang, Y.; Jiang, Q.; Zhang, X. A New Chiral Bis(oxazolinylmethyl)amine Ligand for Ru-Catalyzed Asymmetric Transfer Hydrogenation of Ketones. J. Am. Chem. Soc. 1998, 120, 3817–3818; (b) Xiong, Z.; Pei, C.; Xue, P.; Lv, H.; Zhang, X. Highly enantioselective transfer hydrogenation of racemic alpha-substituted beta-keto sulfonamides via dynamic kinetic resolution. Chem. Commun. 2018, 54, 3883–3886; (c) Xiong, Z.; Tian, J.; Xue, P.; Zhang, X.; Lv, H. Enantioselective synthesis of chiral multicyclic γ-lactones via dynamic kinetic resolution of racemic γ-keto carboxylic acids. Org. Chem. Front. 2020, 7, 104–108.
- 9 Wang, F.; Zheng, L.-S.; Lang, Q.-W.; Yin, C.; Wu, T.; Phansavath, P.; Chen, G.-Q.; Ratovelomanana-Vidal, V.; Zhang, X. Rh(III)-Catalyzed diastereoselective transfer hydrogenation: an efficient entry to key intermediates of HIV protease inhibitors. Chem. Commun. 2020, 56, 3119–3122.
- 10(a) Echeverria, P.-G.; Ferard, C.; Phansavath, P.; Ratovelomanana- Vidal, V. Synthesis, characterization and use of a new tethered Rh(III) complex in asymmetric transfer hydrogenation of ketones. Catal. Commun. 2015, 62, 95–99; (b) Matharu, D. S.; Morris, D. J.; Kawamoto, A. M.; Clarkson, G. J.; Wills, M. A Stereochemically Well-Defined Rhodium(III) Catalyst for Asymmetric Transfer Hydrogenation of Ketones. Org. Lett. 2005, 7, 5489–5491.
- 11 Wang, F.; Yang, T.; Wu, T.; Zheng, L.-S.; Yin, C.; Shi, Y.; Ye, X.-Y.; Chen, G.-Q.; Zhang, X. Asymmetric Transfer Hydrogenation of alpha-Substituted-beta-Keto Carbonitriles via Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2021, 143, 2477–2483.
- 12 Wang, F.; Zhang, Z.; Chen, Y.; Ratovelomanana-Vidal, V.; Yu, P.; Chen, G.-Q.; Zhang, X. Stereodivergent Synthesis of Chiral Succinimides via Rh-Catalyzed Asymmetric Transfer Hydrogenation. Nat. Commun. 2022, 13, 7794.
- 13 Chen, T.; Liu, W.; Gu, W.; Niu, S.; Lan, S.; Zhao, Z.; Gong, F.; Liu, J.; Yang, S.; Cotman, A. E.; Song, J.; Fang, X. Dynamic Kinetic Resolution of β-Substituted α-Diketones via Asymmetric Transfer Hydrogenation. J. Am. Chem. Soc. 2023, 145, 585–599.
- 14 Hashiguchi, S.; Fujii, A.; Takehara, J.; Ikariya, T.; Noyori, R. Asymmetric Transfer Hydrogenation of Aromatic Ketones Catalyzed by Chiral Ruthenium(II) Complexes. J. Am. Chem. Soc. 1995, 117, 7562.
- 15 Murata, K.; Ikariya, T.; Noyori, R. New Chiral Rhodium and Iridium Complexes with Chiral Diamine Ligands for Asymmetric Transfer Hydrogenation of Aromatic Ketones. J. Org. Chem. 1999, 64, 2186–2187.
- 16 Hamada, T.; Torii, T.; Izawa, K.; Noyori, R.; Ikariya, T. Practical Synthesis of Optically Active Styrene Oxides via Reductive Transformation of 2-Chloroacetophenones with Chiral Rhodium Catalysts. Org. Lett. 2002, 4, 4373–4376.
- 17(a) Hayes, A. M.; Morris, D. J.; Clarkson, G. J.; Wills, M. A Class of Ruthenium(II) Catalyst for Asymmetric Transfer Hydrogenations of Ketones. J. Am. Chem. Soc. 2005, 127, 7318–7319; (b) Cheung, F. K.; Hayes, A. M.; Hannedouche, J.; Yim, A. S. Y.; Wills, M. "Tethered" Ru(II) Catalysts for Asymmetric Transfer Hydrogenation of Ketones. J. Org. Chem. 2005, 70, 3188–3197.
- 18 Touge, T.; Nara, H.; Fujiwhara, M.; Kayaki, Y.; Ikariya, T. Efficient Access to Chiral Benzhydrols via Asymmetric Transfer Hydrogenation of Unsymmetrical Benzophenones with Bifunctional Oxo-Tethered Ruthenium Catalysts. J. Am. Chem. Soc. 2016, 138, 10084–10087.
- 19(a) Molina Betancourt, R.; Phansavath, P.; Ratovelomanana-Vidal, V. Rhodium-Catalyzed Asymmetric Transfer Hydrogenation/Dynamic Kinetic Resolution of 3-Benzylidene-Chromanones. Org. Lett. 2021, 23, 1621–1625; (b) Westermeyer, A.; Guillamot, G.; Phansavath, P.; Ratovelomanana-Vidal, V. Synthesis of Enantioenriched β-Hydroxy-γ- Acetal Enamides by Rhodium-Catalyzed Asymmetric Transfer Hydrogenation. Org. Lett. 2020, 22, 3911–3914; (c) Ratovelomanana- Vidal, V.; Phansavath, P.; Molina Betancourt, R.; Echeverria, P.-G.; Ayad, T. Recent Progress and Applications of Transition-Metal-Catalyzed Asymmetric Hydrogenation and Transfer Hydrogenation of Ketones and Imines through Dynamic Kinetic Resolution. Synthesis 2020, 53, 30–50; (d) He, B.; Phansavath, P.; Ratovelomanana-Vidal, V. Rhodium-catalyzed asymmetric transfer hydrogenation of 4-quinolone derivatives. Org. Chem. Front. 2020, 7, 975–979; (e) He, B.; Zheng, L.-S.; Phansavath, P.; Ratovelomanana-Vidal, V. RhIII-Catalyzed Asymmetric Transfer Hydrogenation of α-Methoxy β-Ketoesters through DKR in Water: Toward a Greener Procedure. ChemSusChem 2019, 12, 3032–3036; (f) He, B.; Phansavath, P.; Ratovelomanana-Vidal, V. Rh-Mediated Asymmetric-Transfer Hydrogenation of 3-Substituted Chromones: A Route to Enantioenriched cis-3-(Hydroxymethyl)chroman-4-ol Derivatives through Dynamic Kinetic Resolution. Org. Lett. 2019, 21, 3276–3280; (g) Zheng, L.-S.; Phansavath, P.; Ratovelomanana-Vidal, V. Synthesis of Enantioenriched α,α-Dichloro- and α,α-Difluoro-β-Hydroxy Esters and Amides by Ruthenium-Catalyzed Asymmetric Transfer Hydrogenation. Org. Lett. 2018, 20, 5107–5111; (h) Zheng, L.-S.; Phansavath, P.; Ratovelomanana-Vidal, V. Ruthenium-catalyzed dynamic kinetic asymmetric transfer hydrogenation: stereoselective access to syn 2-(1,2,3,4-tetrahydro-1-isoquinolyl)ethanol derivatives. Org. Chem. Front. 2018, 5, 1366–1370; (i) Zheng, L.-S.; Ferard, C.; Phansavath, P.; Ratovelomanana-Vidal, V. Rhodium-mediated asymmetric transfer hydrogenation: a diastereo- and enantioselective synthesis of syn-α-amido β-hydroxy esters. Chem. Commun. 2018, 54, 283–286; (j) Zheng, L.-S.; Llopis, Q.; Echeverria, P.-G.; Ferard, C.; Guillamot, G.; Phansavath, P.; Ratovelomanana-Vidal, V. Asymmetric Transfer Hydrogenation of (Hetero)arylketones with Tethered Rh(III)-N-(p-Tolylsulfonyl)-1,2-diphenylethylene-1,2-diamine Complexes: Scope and Limitations. J. Org. Chem. 2017, 82, 5607–5615; (k) Ratovelomanana-Vidal, V.; Phansavath, P.; Echeverria, P.-G.; Ayad, T. Recent Developments in Asymmetric Hydrogenation and Transfer Hydrogenation of Ketones and Imines through Dynamic Kinetic Resolution. Synthesis 2016, 48, 2523–2539; (l) Monnereau, L.; Cartigny, D.; Scalone, M.; Ayad, T.; Ratovelomanana-Vidal, V. Efficient Synthesis of Differentiated syn-1,2-Diol Derivatives by Asymmetric Transfer Hydrogenation-Dynamic Kinetic Resolution of α-Alkoxy-Substituted β-Ketoesters. Chem. Eur. J. 2015, 21, 11799–11806.
- 20 Puleo, T. R.; Sujansky, S. J.; Wright, S. E.; Bandar, J. S. Organic Superbases in Recent Synthetic Methodology Research. Chem. Eur. J. 2021, 27, 4216–4229.




