Dcalycinumines A—E, Alkaloids with Cytotoxic Activities of Nasopharyngeal Carcinoma Cells from Daphniphyllum calycinum
Qing Tang
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Q. T., X.-Y. D. and Q. L. contributed equally.
Search for more papers by this authorXiao-Yong Dai
Institute of Biopharmaceutical and Health Engineering, Shenzhen Key Laboratory of Gene and Antibody Therapy, State Key Laboratory of Chemical Oncogenomics, Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Q. T., X.-Y. D. and Q. L. contributed equally.
Search for more papers by this authorQiang Lin
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Q. T., X.-Y. D. and Q. L. contributed equally.
Search for more papers by this authorZhi-Peng Ling
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorYi-Kun Hao
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorTian-Xi Zhu
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorJi-Hui Zhang
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorYi-Bo Hou
Institute of Biopharmaceutical and Health Engineering, Shenzhen Key Laboratory of Gene and Antibody Therapy, State Key Laboratory of Chemical Oncogenomics, Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYao-Lan Li
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorLai-Qiang Huang
Institute of Biopharmaceutical and Health Engineering, Shenzhen Key Laboratory of Gene and Antibody Therapy, State Key Laboratory of Chemical Oncogenomics, Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Jing-Hao Wang
The Guangzhou Key Laboratory of Basic and Translational Research on Chronic Disease, Department of Pharmacy, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, 510630 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guo-Cai Wang
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
The Guangzhou Key Laboratory of Basic and Translational Research on Chronic Disease, Department of Pharmacy, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, 510630 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yu-Bo Zhang
Guangdong Clinical Translational Center for Targeted Drug, Department of Pharmacology, School of Medicine, Jinan University, Guangzhou, Guangdong, 510632 China
The Guangzhou Key Laboratory of Basic and Translational Research on Chronic Disease, Department of Pharmacy, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, 510630 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorQing Tang
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Q. T., X.-Y. D. and Q. L. contributed equally.
Search for more papers by this authorXiao-Yong Dai
Institute of Biopharmaceutical and Health Engineering, Shenzhen Key Laboratory of Gene and Antibody Therapy, State Key Laboratory of Chemical Oncogenomics, Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Q. T., X.-Y. D. and Q. L. contributed equally.
Search for more papers by this authorQiang Lin
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Q. T., X.-Y. D. and Q. L. contributed equally.
Search for more papers by this authorZhi-Peng Ling
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorYi-Kun Hao
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorTian-Xi Zhu
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorJi-Hui Zhang
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorYi-Bo Hou
Institute of Biopharmaceutical and Health Engineering, Shenzhen Key Laboratory of Gene and Antibody Therapy, State Key Laboratory of Chemical Oncogenomics, Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYao-Lan Li
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
Search for more papers by this authorLai-Qiang Huang
Institute of Biopharmaceutical and Health Engineering, Shenzhen Key Laboratory of Gene and Antibody Therapy, State Key Laboratory of Chemical Oncogenomics, Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Jing-Hao Wang
The Guangzhou Key Laboratory of Basic and Translational Research on Chronic Disease, Department of Pharmacy, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, 510630 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Guo-Cai Wang
Institute of Traditional Chinese Medicine & Natural Products, Guangdong Province Key Laboratory of Pharmacodynamic Constituents of TCM and New Drugs Research, College of Pharmacy, Jinan University, Guangzhou, Guangdong, 511443 China
The Guangzhou Key Laboratory of Basic and Translational Research on Chronic Disease, Department of Pharmacy, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, 510630 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yu-Bo Zhang
Guangdong Clinical Translational Center for Targeted Drug, Department of Pharmacology, School of Medicine, Jinan University, Guangzhou, Guangdong, 510632 China
The Guangzhou Key Laboratory of Basic and Translational Research on Chronic Disease, Department of Pharmacy, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, 510630 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Five novel Daphniphyllum alkaloids, named dcalycinumines A—E (1—4, 6), and eight previously described Daphniphyllum alkaloids (5, 7—13) were isolated from Daphniphyllum calycinum. Compound 1 is the first Daphniphyllum alkaloid possessing a highly rearranged 6/6/6/7/5/6 hexacyclic architecture with a unique 3-methyl-1-azabicyclo [4,4,0] decane ring system. Compound 2 represents a rare diamino Daphniphyllum alkaloid with an unprecedented 6/5/5/6/6/5 carbon skeleton featuring a unique 1-aza-6-azaspiro [4,5] decane unit, whereas 3 also represents a rare diamino Daphniphyllum alkaloid as a possible precursor of 2. Compound 4 is the second example of C-22-nor yuzurimine-type alkaloids. Their structures and absolute configurations were elucidated by HRESIMS, NMR spectroscopic analyses, ECD calculations, and single-crystal X-ray diffraction. Moreover, compound 1 showed remarkable antitumor activities, which could inhibit the proliferation, migration and invasion of nasopharyngeal carcinoma cells, and promoted nasopharyngeal carcinoma cells apoptosis.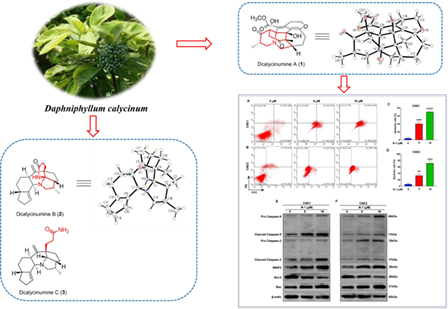
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300442-sup-0001-supinfo.pdfPDF document, 4.5 MB |
Appendix S1: Supporting information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Tang, M. S.; Yang, Y. P.; Tsai, C. C.; Sheue, C. R. The diversity of pistillate flowers and its taxonomic value to the classification of Daphniphyllum (Daphniphyllaceae). Bot. Stud. 2012, 53, 509–524.
- 2 Li, Z. Y.; Guo, Y. W. Progress in the study of Daphniphyllum alkaloids. Chin. J. Org. Chem. 2007, 27, 565–575.
- 3 Chattopadhyay, A. K.; Hanessian, S. Recent progress in the chemistry of Daphniphyllum alkaloids. Chem. Rev. 2017, 117, 4104–4146.
- 4 Guo, L. D.; Chen, Y.; Xu, J. Total synthesis of Daphniphyllum alkaloids: from bicycles to diversified caged structures. Acc. Chem. Res. 2020, 53, 2726–2737.
- 5 Zhang, C. R.; Liu, H. B.; Dong, S. H.; Zhu, J. Y.; Wu, Y.; Yue, J. M. Calycinumines A and B, two novel alkaloids from Daphniphyllum calycinum. Org. Lett. 2009, 11, 4692–4695.
- 6 Di, Y. T.; He, H. P.; Li, C. S.; Tian, J. M.; Mu, S. Z.; Li, S. L.; Gao, S.; Hao, X. J. Yuzurimine-type alkaloids from Daphniphyllum yunnanense. J. Nat. Prod. 2006, 69, 1745–1748.
- 7 Zhang, Q.; Di, Y. T.; Li, C. S.; Fan, X. Tan, C. J.; Zhang, Z.; Zhang, Y.; He, H. P.; Li, S. L.; Hao, X. J. Daphenylline, a new alkaloid with an unusual skeleton, from Daphniphyllum longeracemosum. Org. Lett. 2009, 11, 2357–2359.
- 8 Zhang, Y.; Di, Y. T.; Zhang, Q.; Mu, S. Z.; Tan, C. J.; Fang, X. He, H. P.; Li, S. L.; Hao, X. J. Daphhimalenine A, a new alkaloid with an unprecedented skeleton, from Daphniphyllum himalense. Org. Lett. 2009, 11, 5414–5417.
- 9 Zhang, D. D.; Xu, J. B.; Fan, Y. Y.; Gan, L. S.; Zhang, H.; Yue, J. M. Daphnillonins A and B: alkaloids representing two unknown carbon skeletons from Daphniphyllum longeracemosum. J. Org. Chem. 2020, 85, 3742–3747.
- 10 Zhang, H.; Shyaula, S. L.; Li, J. Y.; Li, J.; Yue, J. M. Himalensines A and B, alkaloids from Daphniphyllum himalense. Org. Lett. 2016, 18, 1202–1205.
- 11 Kobayashi, J. I.; Inaba, Y.; Shiro, M.; Yoshida, N.; Morita, H. Daphnicyclidins A-H, novel hexa- or pentacyclic alkaloids from two species of Daphniphyllum. J. Am. Chem. Soc. 2001, 123, 11402–11408.
- 12 Yamada, R.; Fukuyama, T.; Yokoshima, S. Synthetic studies of the daphniphyllum alkaloids: a cooperative reaction of proximal functional groups forming a tetracyclic system. Org. Lett. 2018, 20, 4504–4506.
- 13 Zhan, Z. J.; Zhang, C. R.; Yue, J. M. Caldaphnidines A–F, six new daphniphyllum alkaloids from Daphniphyllum calycinum. Tetrahedron 2006, 65, 11038–11045.
- 14 Zhang, C. R.; Yang, S. P.; Yue, J. M. Alkaloids from the twigs of Daphniphyllum calycinum. J. Nat. Prod. 2008, 71, 1663–1668.
- 15 Li, Z. Y.; Peng, S. Y.; Fang, L.; Yang, Y. M.; Guo, Y. W. Two new daphniphyllum alkaloids from the stems and leaves of Daphniphyllum calycinum. Chem. Biodivers. 2009, 6, 105–110.
- 16 Shen, S. M.; Li, H.; Wang, J. R.; Zeng, Y. B.; Guo, Y. W. Further new complex daphniphyllum alkaloids from the stems and leaves of Daphniphyllum calycinum: structure and stereochemistry. Tetrahedron 2020, 76, 131616.
- 17 Yang, J.; Liu, X.; Fu, J.; Lyu, H. Y.; Bai, L. P.; Jiang, Z. H.; Zhu, G. Y. Calycindaphines A–J, daphniphyllum alkaloids from the roots of Daphniphyllum calycinum. RSC Adv. 2021, 11, 9057–9066.
- 18 Yang, S. P.; Yue, J. M. Two novel alkaloids with a unique fused hexacyclic skeleton from Daphniphyllum subverticillatum. J. Org. Chem. 2003, 68, 7961–7966.
- 19
Nakano, T.; Saeki, Y. Daphniphyllum alkaloids. Part II. The isolation and the structures of the alkaloids from Daphniphyllum macropodum miquel. Tetrahedron Lett. 1967, 8, 4791–4797.
10.1016/S0040-4039(01)89605-2 Google Scholar
- 20 Du, C.; Fang, J. Chen, J.; Liu, Z.; Li, H.; Wang, X.; Xie, X.; She, X. Construction of the tetracyclic core of calyciphylline B-type daphniphyllum alkaloids. Org. Lett. 2019, 21, 8718–8721.
- 21 Luo, D.; Chen, N. H.; Wang, W. Z.; Zhang, J. H.; Li, C. J.; Zhuo, X. F.; Tu, Z. C.; Wu, Z. N.; Fan, C. L.; Zhang, H. P.; Li, Y. L.; Wang, G. C.; Zhang, Y. B. Chin. J. Chem. 2021, 39, 3339–3346.
- 22 Luo, D.; Wu, Z. N.; Zhang, J. H.; Lin, Q.; Chen, N. H.; Chen, S.; Tang, Q.; Zhan, Z. C.; Fan, C. L.; Li, Y. L.; Wang, G. C.; Zhang, Y. B. Sophaloseedlines A–G: diverse matrine-based alkaloids from Sophora alopecuroides with potential anti-hepatitis B virus activities. Chin. J. Chem. 2021, 39, 2555–2562.




