In-situ Generation of Hydroxyl Layers in CoO@FeSe2 Catalyst for High Selectivity Seawater Electrolysis
Suyang Feng
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorPeng Rao
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorXiao Wu
National Energy Group Ledong Power Generation Co., Ltd., Ledong, Hainan, 572539 China
Search for more papers by this authorKe Li
National Energy Group Ledong Power Generation Co., Ltd., Ledong, Hainan, 572539 China
Search for more papers by this authorAnyuan Qi
National Energy Group Ledong Power Generation Co., Ltd., Ledong, Hainan, 572539 China
Search for more papers by this authorYanhui Yu
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorCorresponding Author
Jing Li
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorPeilin Deng
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorYuliang Yuan
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorCorresponding Author
Shaolei Wang
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, School of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorCorresponding Author
Xinlong Tian
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorCorresponding Author
Zhenye Kang
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorSuyang Feng
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorPeng Rao
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorXiao Wu
National Energy Group Ledong Power Generation Co., Ltd., Ledong, Hainan, 572539 China
Search for more papers by this authorKe Li
National Energy Group Ledong Power Generation Co., Ltd., Ledong, Hainan, 572539 China
Search for more papers by this authorAnyuan Qi
National Energy Group Ledong Power Generation Co., Ltd., Ledong, Hainan, 572539 China
Search for more papers by this authorYanhui Yu
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorCorresponding Author
Jing Li
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorPeilin Deng
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorYuliang Yuan
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
Search for more papers by this authorCorresponding Author
Shaolei Wang
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, School of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorCorresponding Author
Xinlong Tian
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorCorresponding Author
Zhenye Kang
State Key Laboratory of Marine Resource Utilization in South China Sea, Hainan Provincial Key Lab of Fine Chemistry, School of Chemical Engineering and Technology, Hainan University, Haikou, Hainan, 570228 China
E-mail: [email protected] (J. Li); [email protected] (S. Wang); [email protected] (X. Tian); [email protected] (Z. Kang);Search for more papers by this authorComprehensive Summary
Seawater electrolysis holds great promise for hydrogen production in the future, while the development of anodic catalysts has been severely hampered by the side-reaction, chloride evolution reaction. In this work, nano-flower-cluster structured CoO@FeSe2/CF catalysts are synthesized via a scalable electrodeposition technique, and the performance is systematically studied. The oxygen evolution reaction (OER) overpotential of CoO@FeSe2/CF is 267 mV at 100 mA·cm−2, which is significantly lower than that of the IrO2 catalyst (435 mV). Additionally, the catalyst shows high selectivity for OER (97.9%) and almost no loss of activity after a durability test for 1100 h. The impressive performance is attributed to the dense rod-like structure with abundant active centers after electrochemical activation and the in-situ generated CoOOH and FeOOH that improve the catalytic activity of the catalyst. The synergistic effect induced by the non-uniform structure endows the catalyst with excellent stability.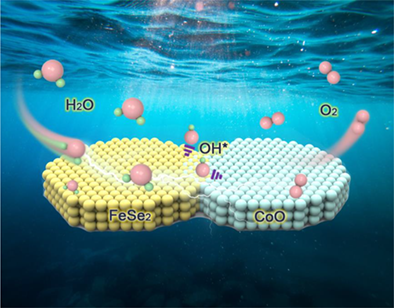
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300441-sup-0001-supinfo.pdfPDF document, 1.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Tian, X.; Lu, X. F.; Xia, B. Y.; Lou, X. W. D. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies. Joule 2020, 4, 45–68.
- 2 Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E. J.; Lou, X. W. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856.
- 3 Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 2021, 31, 2006484.
- 4 Guo, J.; Zheng, Y.; Hu, Z.; Zheng, C.; Mao, J.; Du, K.; Jaroniec, M.; Qiao, S.-Z.; Ling, T. Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 2023, 8, 264–272.
- 5
Ma, T.; Xu, W.; Li, B.; Chen, X.; Zhao, J.; Wan, S.; Jiang, K.; Zhang, S.; Wang, Z.; Tian, Z. The critical role of additive sulfate for stable alkaline seawater oxidation on nickel-based electrodes. Angew. Chem. 2021, 133, 22922–22926.
10.1002/ange.202110355 Google Scholar
- 6
Gao, M.; Zhou, W.-Y.; Mo, Y.-X.; Sheng, T.; Deng, Y.; Chen, L.; Wang, K.; Tan, Y.; Zhou, H. Outstanding long-cycling lithium-sulfur batteries by core-shell structure of S@Pt composite with ultrahigh sulfur content. Adv. Powder Mater. 2022, 1, 100006.
10.1016/j.apmate.2021.09.006 Google Scholar
- 7 Park, Y. S.; Jeong, J.-Y.; Jang, M. J.; Kwon, C.-Y.; Kim, G. H.; Jeong, J.; Lee, J.-h.; Lee, J.; Choi, S. M. Ternary layered double hydroxide oxygen evolution reaction electrocatalyst for anion exchange membrane alkaline seawater electrolysis. J. Energy Chem. 2022, 75, 127–134.
- 8 Arulmozhi, N.; Hanselman, S.; Tudor, V.; Chen, X.; van Velden, D.; Schneider, G. F.; Calle-Vallejo, F.; Koper, M. T. Energetics and kinetics of hydrogen electrosorption on a graphene-covered Pt (111) electrode. JACS Au 2023, 3, 526–535.
- 9 Rao, P.; Deng, Y.; Fan, W.; Luo, J.; Deng, P.; Li, J.; Shen, Y.; Tian, X. Movable type printing method to synthesize high-entropy single-atom catalysts. Nat. Commun. 2022, 13, 5071.
- 10 Zhao, R.; Chen, Z.; Li, Q.; Wang, X.; Tang, Y.; Fu, G.; Li, H.; Lee, J.-M.; Huang, S. N-doped LaPO4: An effective Pt-free catalyst for electrocatalytic oxygen reduction. Chem. Catal. 2022, 2, 3590–3606.
- 11 Wang, X.; Wang, J.; Wang, P.; Li, L.; Zhang, X.; Sun, D.; Li, Y.; Tang, Y.; Wang, Y.; Fu, G. Engineering 3d-2p-4f gradient orbital coupling to enhance electrocatalytic oxygen reduction. Adv. Mater. 2022, 34, 2206540.
- 12 Gao, Y.; Xue, Y.; He, F.; Li, Y. Controlled growth of a high selectivity interface for seawater electrolysis. PANS 2022, 119, e2206946119.
- 13 Tran, P. K. L.; Tran, D. T.; Malhotra, D.; Prabhakaran, S.; Kim, D. H.; Kim, N. H.; Lee, J. H. Highly effective freshwater and seawater electrolysis enabled by atomic Rh-modulated Co-CoO lateral heterostructures. Small 2021, 17, 2103826.
- 14 Lyu, C.; Cao, C.; Cheng, J.; Yang, Y.; Wu, K.; Wu, J.; Lau, W.-M.; Qian, P.; Wang, N.; Zheng, J. Interfacial electronic structure modulation of Ni2P/Ni5P4 heterostructure nanosheets for enhanced pH-universal hydrogen evolution reaction performance. Chem. Eng. J. 2023, 464, 142538.
- 15 Yang, C.; Zhou, L.; Wang, C.; Duan, W.; Zhang, L.; Zhang, F.; Zhang, J.; Zhen, Y.; Gao, L.; Fu, F. Large-scale synthetic Mo@(2H-1T)-MoSe2 monolithic electrode for efficient hydrogen evolution in all pH scale ranges and seawater. Appl. Catal., B 2022, 304, 120993.
- 16 Li, R.; Li, Y.; Yang, P.; Ren, P.; Wang, D.; Lu, X.; Xu, R.; Li, Y.; Xue, J.; Zhang, J. Synergistic interface engineering and structural optimization of non-noble metal telluride-nitride electrocatalysts for sustainably overall seawater electrolysis. Appl. Catal., B 2022, 318, 121834.
- 17 Xu, W.; Fan, G.; Zhu, S.; Liang, Y.; Cui, Z.; Li, Z.; Jiang, H.; Wu, S.; Cheng, F. Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2107333.
- 18 Wang, H.-Y.; Ren, J.-T.; Wang, L.; Sun, M.-L.; Yang, H.-M.; Lv, X.-W.; Yuan, Z.-Y. Synergistically enhanced activity and stability of bifunctional nickel phosphide/sulfide heterointerface electrodes for direct alkaline seawater electrolysis. J. Energy Chem. 2022, 75, 66–73.
- 19 Yu, Z.; Li, Y.; Martin-Diaconescu, V.; Simonelli, L.; Ruiz Esquius, J.; Amorim, I.; Araujo, A.; Meng, L.; Faria, J. L.; Liu, L. Highly Efficient and Stable Saline Water Electrolysis Enabled by Self-Supported Nickel-Iron Phosphosulfide Nanotubes With Heterointerfaces and Under-Coordinated Metal Active Sites. Adv. Funct. Mater. 2022, 32, 2206138.
- 20 Mao, Q.; Deng, K.; Yu, H.; Xu, Y.; Wang, Z.; Li, X.; Wang, L.; Wang, H. In situ reconstruction of partially hydroxylated porous Rh metallene for ethylene glycol-assisted seawater splitting. Adv. Funct. Mater. 2022, 32, 2201081.
- 21 Li, Y.; Zhang, Q.; Zhao, X.; Wu, H.; Wang, X.; Zeng, Y.; Chen, Q.; Chen, M.; Liu, P. Vapor Phase Dealloying Derived Nanoporous Co@CoO/RuO2 Composites for Efficient and Durable Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2214124.
- 22 Wang, L.; Pan, Y.; Wu, D.; Liu, X.; Cao, L.; Zhang, W.; Chen, H.; Liu, T.; Liu, D.; Chen, T. The in situ formation of defective CoOOH catalysts from semi-oxidized Co for alkaline oxygen evolution reaction. J. Mater. Chem. A 2022, 10, 20011–20017.
- 23 Fan, C.; Wang, X.; Wu, X.; Chen, Y.; Wang, Z.; Li, M.; Sun, D.; Tang, Y.; Fu, G. Neodymium-evoked valence electronic modulation to balance reversible oxygen electrocatalysis. Adv. Energy Mater. 2023, 13, 2203244.
- 24 Liao, H.; Zhang, X.; Niu, S.; Tan, P.; Chen, K.; Liu, Y.; Wang, G.; Liu, M.; Pan, J. Dynamic dissolution and re-adsorption of molybdate ion in iron incorporated nickel-molybdenum oxyhydroxide for promoting oxygen evolution reaction. Appl. Catal., B 2022, 307, 121150.
- 25 Rao, P.; Wu, D.; Wang, T.-J.; Li, J.; Deng, P.; Chen, Q.; Shen, Y.; Chen, Y.; Tian, X. Single atomic cobalt electrocatalyst for efficient oxygen reduction reaction. eScience 2022, 2, 399–404.
- 26 Li, M.; Feng, L. G. NiSe2-CoSe2 with a Hybrid Nanorods and Nanoparticles Structure for Efficient Oxygen Evolution Reaction. Chin. J. Struct. Chem. 2022, 41, 2201019–2201024.
- 27 Hedenstedt, K.; Bäckström, J.; Ahlberg, E. In-situ Raman spectroscopy of α-and γ-FeOOH during cathodic load. J. Electrochem. Soc. 2017, 164, H621.
- 28 Moysiadou, A.; Lee, S.; Hsu, C.-S.; Chen, H. M.; Hu, X. Mechanism of oxygen evolution catalyzed by cobalt oxyhydroxide: cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining step. J. Am. Chem. Soc. 2020, 142, 11901–11914.
- 29
Jing, H.; Zhu, P.; Zheng, X.; Zhang, Z.; Wang, D.; Li, Y. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder. Mater. 2022, 1, 100013.
10.1016/j.apmate.2021.10.004 Google Scholar
- 30 Yu, L.; Xiao, J.; Huang, C.; Zhou, J.; Qiu, M.; Yu, Y.; Ren, Z.; Chu, C.-W.; Yu, J. C. High-performance seawater oxidation by a homogeneous multimetallic layered double hydroxide electrocatalyst. PANS 2022, 119, e2202382119.
- 31 Yang, Y.; Su, J.; Jiang, P.; Chen, J.; Hu, L.; Chen, Q. MOFs-Derived N-Doped Carbon-Encapsulated Metal/Alloy Electrocatalysts to Tune the Electronic Structure and Reactivity of Carbon Active Sites. Chin. J. Chem. 2021, 39, 2626–2637.
- 32 Sun, W.; Wei, Z.; Qi, J.; Kang, L.; Li, J.; Xie, J.; Tang, B.; Xie, Y. Rapid and scalable synthesis of Prussian blue analogue nanocubes for electrocatalytic water oxidation. Chin. J. Chem. 2021, 39, 2347–2353.
- 33 Rao, P.; Wu, D.; Luo, J.; Li, J.; Deng, P.; Shen, Y.; Tian, X. A plasma bombing strategy to synthesize high-loading single-atom catalysts for oxygen reduction reaction. Cell Rep. Phys. Sci. 2022, 3, 100880.
- 34 Li, M.; Feng, L.; NiSe2-CoSe2 with a hybrid nanorods and nanoparticles structure for efficient oxygen evolution reaction. Chin. J. Struct. Chem. 2022, 41, 2201019–2201024.
- 35 Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 2016, 352, 333–337.
- 36 Liao, L.; Yang, L.; Zhao, G.; Zhou, H.; Cai, F.; Li, Y.; Wang, X.; Yu, F. Boosting pH-Universal Hydrogen Evolution of Molybdenum Disulfide Particles by Interfacial Engineering. Chin. J. Chem. 2021, 39, 288–294.
- 37 Li, G.; Feng, S.; Li, J.; Deng, P.; Tian, X.; Wang, C.; Hua, Y. P-Ni4Mo Catalyst for Seawater Electrolysis with High Current Density and Durability. Chin. J. Struct. Chem. 2022, 41, 2207068–2207073.
- 38 Lin, Y.; Chen, X.; Tuo, Y.; Pan, Y.; Zhang, J. In-situ doping-induced lattice strain of NiCoP/S nanocrystals for robust wide pH hydrogen evolution electrocatalysis and supercapacitor. J. Energy Chem. 2022, 70, 27–35.
- 39 Jiang, S.; Liu, Y.; Qiu, H.; Su, C.; Shao, Z. High selectivity electrocatalysts for oxygen evolution reaction and anti-chlorine corrosion strategies in seawater splitting. Catalysts 2022, 12, 261.
- 40 Yu, M.; Li, J.; Liu, F.; Liu, J.; Xu, W.; Hu, H.; Chen, X.; Wang, W.; Cheng, F. Anionic formulation of electrolyte additive towards stable electrocatalytic oxygen evolution in seawater splitting. J. Energy Chem. 2022, 72, 361–369.
- 41 Yang, J.; Jin, X.; Cheng, Z.; Zhou, H.; Gao, L.; Jiang, D.; Jie, X.; Ma, Y.; Chen, W. Facile and green synthesis of bifunctional carbon dots for detection of Cu2+ and ClO– in aqueous solution. ACS Sustainable Chem. Eng. 2021, 9, 13206–13214.
- 42 Cui, B.; Hu, Z.; Liu, C.; Liu, S.; Chen, F.; Hu, S.; Zhang, J.; Zhou, W.; Deng, Y.; Qin, Z. Heterogeneous lamellar-edged Fe-Ni (OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2021, 14, 1149–1155.
- 43 Liu, H.; Lei, J.; Yang, S.; Qin, F.; Cui, L.; Kong, Y.; Zheng, X.; Duan, T.; Zhu, W.; He, R. Boosting the oxygen evolution activity over cobalt nitride nanosheets through optimizing the electronic configuration. Appl. Catal., B 2021, 286, 119894.
- 44 Wang, X.; Wang, J.; Yu, B.; Jiang, W.; Wei, J.; Chen, B.; Xu, R.; Yang, L. Facile synthesis MnCo2O4.5@C nanospheres modifying PbO2 energy-saving electrode for zinc electrowinning. J. Hazard. Mater. 2022, 428, 128212.
- 45 Feng, S.; Wang, J.; Wang, W.; Wang, X.; Zhang, Y.; Ju, A.; Pan, J.; Xu, R. The Ni-Mo-S Catalyst@ Copper Foams with Excellent Stability and 1.5 V Drive Electrolytic Water. Adv. Mater. Interfaces 2021, 8, 2100500.
- 46 Wang, X.; Wang, J.; Jiang, W.; Chen, C.; Yu, B.; Xu, R. Facile synthesis MnCo2O4 modifying PbO2 composite electrode with enhanced OER electrocatalytic activity for zinc electrowinning. Sep. Purif. Technol. 2021, 272, 118916.
- 47 Liu, Z.; Yu, X.; Xue, H.; Feng, L. A nitrogen-doped CoP nanoarray over 3D porous Co foam as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2019, 7, 13242–13248.




