Nickel-Catalyzed Regioselective Hydrosilylation of Conjugated Dienes†
Xiaoyu Wu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorWei Liu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorLiqun Yang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorYue Wang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorTianwen Liu
Joseph Black Building, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ UK
Search for more papers by this authorYao Yuan
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorYan Lu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Zhaoguo Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
E-mail: [email protected]Search for more papers by this authorXiaoyu Wu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorWei Liu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorLiqun Yang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorYue Wang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorTianwen Liu
Joseph Black Building, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ UK
Search for more papers by this authorYao Yuan
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorYan Lu
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Zhaoguo Zhang
Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
With the increasing demand for homoallylic silanes and allylic silanes, the highly efficient and regioselective hydrosilylations of conjugated dienes are urgently needed. Herein, we developed a Ni-catalyzed regiodivergent hydrosilylation of aromatic conjugated dienes by adjusting the temperature and ligands. Under low temperature (–30 °C), an eternal-ligand-free system (Ni/t-BuOK) can efficiently facilitate the 3,4-anti-Markovnikov hydrosilylation to provide homoallylic silanes via electrophilic activation process; under room temperature (25 °C), a ligand-controlled system (Ni/t-BuOK/PPh3) can eventuate the 3,4-Markovnikov hydrosilylation to produce allylic silanes via Chalk-Harrod process. Both systems are compatible with various conjugated dienes and primary silanes in excellent yields and regioselectivities.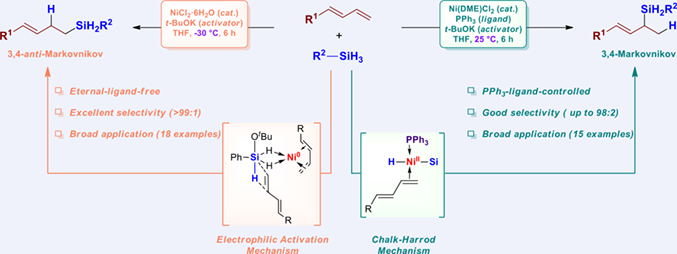
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300407-sup-0001-supinfo.pdfPDF document, 2.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Chabaud, L.; James, P.; Landais, Y. Allylsilanes in Organic Synthesis − Recent Developments. Eur. J. Org. Chem. 2004, 2004, 3173−3199; (b) Denmark, S. E.; Werner, N. S. Cross-Coupling of Aromatic Bromides with Allylic Silanolate Salts. J. Am. Chem. Soc. 2008, 130, 16382−16393; (c) Cheng, Z.; Li, M.; Zhang, X.-Y.; Sun, Y.; Yu, Q.-L.; Zhang, X.-H.; Lu, Z. Cobalt-Catalyzed Regiodivergent Double Hydrosilylation of Arylacetylenes. Angew. Chem. Int. Ed. 2023, 62, e202215029; (d) Chen, J.; Deng, G.; Wang, Y.; Zhu, S. Facile Synthesis of Chiral α-Hydroxy Ketones by a Ni-Catalyzed Multicomponent Hydrometallation–CO Insertion–Enantioconvergent Alkylation Cascade. Chin. J. Chem. 2023, 41, 294−300.
- 2(a) Rappoport, Z.; Apeloig, Y. The Chemistry of Organic Silicon Compounds , Wiley, 2001, pp. 1−1479; (b) Obligacion, J. V.; Chirik, P. J. Earth-Abundant Transition Metal Catalysts for Alkene Hydrosilylation and Hydroboration. Nat. Rev. Chem. 2018, 2, 15−34; (c) Chen, J.; Guo, J.; Lu, Z. Recent Advances in Hydrometallation of Alkenes and Alkynes via the First Row Transition Metal Catalysis. Chin. J. Chem. 2018, 36, 1075−1109; (d) Guo, J.; Cheng, Z.; Chen, J.; Chen, X.; Lu, Z. Iron- and Cobalt-Catalyzed Asymmetric Hydrofunctionalization of Alkenes and Alkynes. Acc. Chem. Res. 2021, 54, 2701−2716.
- 3(a) Ikeda, S.-i. Nickel-Catalyzed Coupling of Carbonyl Compounds and Alkynes or 1,3-Dienes: An Efficient Method for the Preparation of Allylic, Homoallylic, and Bishomoallylic Alcohols. Angew. Chem. Int. Ed. 2003, 42, 5120−5122;
(b) Kimura, M.; Tamaru, Y. Reaction of Dienes and Allenes. In Modern Organonickel Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2005, pp. 137−170;
10.1002/3527604847.ch5 Google Scholar(c) McNeill, E.; Ritter, T. 1,4-Functionalization of 1,3-Dienes with Low-Valent Iron Catalysts. Acc. Chem. Res. 2015, 48, 2330–2343; (d) Wu, Z.; Zhang, W. Recent Advances in Metal-Catalyzed 1,2-Difunctionalization of Conjugated Dienes. Chin. J. Org. Chem. 2017, 37, 2250−2262; (e) Li, G.; Huo, X.; Jiang, X.; Zhang, W. Asymmetric Synthesis of Allylic Compounds via Hydrofunctionalisation and Difunctionalisation of Dienes, Allenes, and Alkynes. Chem. Soc. Rev. 2020, 49, 2060−2118; (f) Rocard, L.; Chen, D.; Stadler, A.; Zhang, H.; Gil, R.; Bezzenine, S.; Hannedouche, J. Earth-Abundant 3d Transition Metal Catalysts for Hydroalkoxylation and Hydroamination of Unactivated Alkenes. Catalysts 2021, 11, 674.
- 4(a) Bailey, D. L.; Pines, A. N. Reactions of Allylic Silicon Compounds. Ind. Eng. Chem. 1954, 46, 2363−2367;
(b) Shiihara, I.; Hoskyns, W. F.; Post, H. W. Studies in Organosilicon Chemistry. XXXIX. The Addition of Silanes to Isoprene. J. Org. Chem. 1961, 26, 4000−4002;
(c) Kiso, Y.; Yamamoto, K.; Tamao, K.; Kumada, M. Asymmetric Homogeneous Hydrosilylation with Chiral Phosphine-Palladium Complexes. J. Am. Chem. Soc. 1972, 94, 4373−4374;
(d) Cornish, A. J.; Lappert, M. F.; Nile, T. A. Homogenous Catalysis: V. Hydrosilyation of Dienes with Octacarbonyldicobalt(0). J. Organomet. Chem. 1977, 136, 73−85;
(e) Ojila, I.; Kumagai, M. Regioselective Hydrosilylation of Isoprene Catalyzed by Tris(Triphenylphosphine)Chlororhodium. J. Organomet. Chem. 1977, 134, C6−C10;
(f) Hilt, G.; Lüers, S.; Schmidt, F. Cobalt(I)- Catalyzed Diels-Alder, 1,4-Hydrovinylation and 1,4-Hydrosilylation Reactions of Non-Activated Starting Materials on a Large Scale. Synthesis 2004, 2004, 634−638;
10.1055/s-2003-44373 Google Scholar(g) Bareille, L.; Becht, S.; Cui, J. L.; Le Gendre, P.; Moïse, C. First Titanium-Catalyzed anti-1,4-Hydrosilylation of Dienes. Organometallics 2005, 24, 5802−5806; (h) Han, J. W.; Hayashi, T. Palladium-catalyzed asymmetric hydrosilylation of 1,3-dienes. Tetrahedron: Asymmetry 2010, 21, 2193−2197; (i) Wu, J. Y.; Stanzl, B. N.; Ritter, T. A Strategy for the Synthesis of Well-Defined Iron Catalysts and Application to Regioselective Diene Hydrosilylation. J. Am. Chem. Soc. 2010, 132, 13214−13216; (j) Gendre, P. L.; Pop, R.; Cui, J.; Adriaenssens, L.; Comte, V. [Me2C(C5H4)2TiMe2]: An Open- Bent Titanocene Catalyst for the Hydrosilylation of Bulky 1,3-Dienes. Synlett 2011, 2011, 679−683;10.1055/s-0030-1259687 Google Scholar(k) Park, H. S.; Han, J. W.; Shintani, R.; Hayashi, T. Asymmetric Hydrosilylation of Cyclohexa-1,3-Diene with Trichlorosilane by Palladium Catalysts Coordinated with Chiral Phosphoramidite Ligands. Tetrahedron: Asymmetry 2013, 24, 418−420; (l) Srinivas, V.; Nakajima, Y.; Ando, W.; Sato, K.; Shimada, S. ChemInform Abstract: Bis(acetylacetonato)Ni(II)/NaBHEt3-Catalyzed Hydrosilylation of 1,3-Dienes, Alkenes and Alkynes. J. Organomet. Chem. 2016, 809, 57−62; (m) Kuai, C.-S.; Ji, D.-W.; Zhao, C.-Y.; Liu, H.; Hu, Y.-C.; Chen, Q.-A. Ligand-Regulated Regiodivergent Hydrosilylation of Isoprene under Iron Catalysis. Angew. Chem. Int. Ed. 2020, 59, 19115−19120; (n) Yang, S.-N.; Liu, C.-H.; He, L.-B.; Zheng, H.; Kuai, C.-S.; Wan, B.; Ji, D.-W.; Chen, Q.-A. Ligand-Controlled Regiodivergence in Cobalt-Catalyzed Hydrosilylation of Isoprene. Org. Chem. Front. 2023, 10, 2204−2210.
- 5(a) Greenhalgh, M. D.; Frank, D. J.; Thomas, S. P. Iron-Catalysed Chemo-, Regio-, and Stereoselective Hydrosilylation of Alkenes and Alkynes Using a Bench-Stable Iron(II) Pre-Catalyst. Adv. Synth. Catal. 2014, 356, 584−590; (b) Hu, M.-Y.; He, Q.; Fan, S.-J.; Wang, Z.-C.; Liu, L.-Y.; Mu, Y.-J.; Peng, Q.; Zhu, S.-F. Ligands with 1,10-Phenanthroline Scaffold for Highly Regioselective Iron-Catalyzed Alkene Hydrosilylation. Nat. Commun. 2018, 9, 221; (c) Sang, H. L.; Yu, S.; Ge, S. Cobalt-Catalyzed Regioselective Stereoconvergent Markovnikov 1,2-Hydrosilylation of Conjugated Dienes. Chem. Sci. 2018, 9, 973−978; (d) Wen, H.; Wang, K.; Zhang, Y.; Liu, G.; Huang, Z. Cobalt-Catalyzed Regio- and Enantioselective Markovnikov 1,2-Hydrosilylation of Conjugated Dienes. ACS Catal. 2019, 9, 1612−1618; (e) Komine, N.; Mitsui, T.; Kikuchi, S.; Hirano, M. Ligand-Controlled Regiodivergent Hydrosilylation of Conjugated Dienes Catalyzed by Mono(phosphine)palladium(0) Complexes. Organometallics 2020, 39, 4510−4524; (f) Wang, Z.-L.; Wang, Y.; Xu, J.-L.; Zhao, M.; Dai, K.-Y.; Shan, C.-C.; Xu, Y.-H. Synthesis of Structurally Diverse Allylsilanes via Copper-Catalyzed Regiodivergent Hydrosilylation of 1,3-Dienes. Org. Lett. 2021, 23, 4736−4742; (g) Wang, Y.; Wang, Z.-L.; Ma, W.-W.; Xu, Y.-H. Copper- Catalyzed Markovnikov Selective 3,4-Hydrosilylation of 2-Substituted 1,3-Dienes. Org. Lett. 2022, 24, 4081−4086; (h) Wang, L.; Lu, W.; Zhang, J.; Chong, Q.; Meng, F. Cobalt-Catalyzed Regio-, Diastereo- and Enantioselective Intermolecular Hydrosilylation of 1,3-Dienes with Prochiral Silanes. Angew. Chem. Int. Ed. 2022, 61, e202205624; (i) Qi, L.; Pan, Q.-Q.; Wei, X.-X.; Pang, X.; Liu, Z.; Shu, X.-Z. Nickel-Catalyzed Reductive [4 + 1] Sila-Cycloaddition of 1,3-Dienes with Dichlorosilanes. J. Am. Chem. Soc. 2023, 145, 13008−13014.
- 6 Parker, S. E.; Börgel, J.; Ritter, T. 1,2-Selective Hydrosilylation of Conjugated Dienes. J. Am. Chem. Soc. 2014, 136, 4857−4860.
- 7(a) Ibrahim, A. D.; Entsminger, S. W.; Zhu, L.; Fout, A. R. A Highly Chemoselective Cobalt Catalyst for the Hydrosilylation of Alkenes using Tertiary Silanes and Hydrosiloxanes. ACS Catal. 2016, 6, 3589−3593; (b) Raya, B.; Jing, S.; Balasanthiran, V.; RajanBabu, T. V. Control of Selectivity through Synergy between Catalysts, Silanes, and Reaction Conditions in Cobalt-Catalyzed Hydrosilylation of Dienes and Terminal Alkenes. ACS Catal. 2017, 7, 2275−2283.
- 8 Sun, W.; Li, M.-P.; Li, L.-J.; Huang, Q.; Hu, M.-Y.; Zhu, S.-F. Phenanthroline-Imine Ligands for Iron-Catalyzed Alkene Hydrosilylation. Chem. Sci. 2022, 13, 2721−2728.
- 9(a) Calimano, E.; Tilley, T. D. Alkene Hydrosilation by a Cationic Hydrogen-Substituted Iridium Silylene Complex. J. Am. Chem. Soc. 2008, 130, 9226−9227; (b) Fasulo, M. E.; Lipke, M. C.; Tilley, T. D. Structural and Mechanistic Investigation of a Cationic Hydrogen-Substituted Ruthenium Silylene Catalyst for Alkene Hydrosilation. Chem. Sci. 2013, 4, 3882−3887; (c) Lipke, M. C.; Liberman-Martin, A. L.; Tilley, T. D. Electrophilic Activation of Silicon–Hydrogen Bonds in Catalytic Hydrosilations. Angew. Chem. Int. Ed. 2017, 56, 2260−2294; (d) Lipke, M. C.; Poradowski, M.-N.; Raynaud, C.; Eisenstein, O.; Tilley, T. D. Catalytic Olefin Hydrosilations Mediated by Ruthenium η3-H2Si σ Complexes of Primary and Secondary Silanes. ACS Catal. 2018, 8, 11513−11523; (e) Smith, P. W.; Dong, Y.; Tilley, T. D. Efficient and Selective Alkene Hydrosilation Promoted by Weak, Double Si–H Activation at an Iron Center. Chem. Sci. 2020, 11, 7070−7075.
- 10 Wu, X.; Ding, G.; Lu, W.; Yang, L.; Wang, J.; Zhang, Y.; Xie, X.; Zhang, Z. Nickel-Catalyzed Hydrosilylation of Terminal Alkenes with Primary Silanes via Electrophilic Silicon–Hydrogen Bond Activation. Org. Lett. 2021, 23, 1434−1439.
- 11(a) Banerjee, S.; Yang, Y.-F.; Jenkins, I. D.; Liang, Y.; Toutov, A. A.; Liu, W.-B.; Schuman, D. P.; Grubbs, R. H.; Stoltz, B. M.; Krenske, E. H.; Houk, K. N.; Zare, R. N. Ionic and Neutral Mechanisms for C–H Bond Silylation of Aromatic Heterocycles Catalyzed by Potassium tert-Butoxide. J. Am. Chem. Soc. 2017, 139, 6880−6887; (b) Docherty, J. H.; Peng, J.; Dominey, A. P.; Thomas, S. P. Activation and Discovery of Earth-Abundant Metal Catalysts Using Sodium tert-Butoxide. Nat. Chem. 2017, 9, 595−600; (c) Liu, W.-B.; Schuman, D. P.; Yang, Y.-F.; Toutov, A. A.; Liang, Y.; Klare, H. F. T.; Nesnas, N.; Oestreich, M.; Blackmond, D. G.; Virgil, S. C.; Banerjee, S.; Zare, R. N.; Grubbs, R. H.; Houk, K. N.; Stoltz, B. M. Potassium tert-Butoxide-Catalyzed Dehydrogenative C–H Silylation of Heteroaromatics: A Combined Experimental and Computational Mechanistic Study. J. Am. Chem. Soc. 2017, 139, 6867−6879; (d) Asgari, P.; Hua, Y.; Bokka, A.; Thiamsiri, C.; Prasitwatcharakorn, W.; Karedath, A.; Chen, X.; Sardar, S.; Yum, K.; Leem, G.; Pierce, B. S.; Nam, K.; Gao, J.; Jeon, J. Catalytic Hydrogen Atom Transfer from Hydrosilanes to Vinylarenes for Hydrosilylation and Polymerization. Nat. Catal. 2019, 2, 164−173; (e) Jenkins, I. D.; Krenske, E. H. Mechanistic Aspects of Hydrosilane/Potassium tert-Butoxide (HSiR3/KOtBu)-Mediated Reactions. ACS Omega 2020, 5, 7053−7058.
- 12(a) Nattmann, L.; Saeb, R.; Nöthling, N.; Cornella, J. An Air-Stable Binary Ni(0)–Olefin Catalyst. Nat. Catal. 2020, 3, 6−13; (b) Tran, V. T.; Li, Z.-Q.; Apolinar, O.; Derosa, J.; Joannou, M. V.; Wisniewski, S. R.; Eastgate, M. D.; Engle, K. M. Ni(COD)(DQ): An Air-Stable 18-Electron Nickel(0)–Olefin Precatalyst. Angew. Chem. Int. Ed. 2020, 59, 7409−7413.




