A Comprehensive Review on Mechanisms and Applications of Rare-Earth Based Perovskite Nanocrystals†
Xiaoshan Zhang
Academy for Engineering and Technology, Institute of Future Lighting, Fudan University, Shanghai, 200433 China
Search for more papers by this authorYikun Wang
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorXiang Wu
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorFeilong Wang
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorQiongrong Ou
Academy for Engineering and Technology, Institute of Future Lighting, Fudan University, Shanghai, 200433 China
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Shuyu Zhang
Academy for Engineering and Technology, Institute of Future Lighting, Fudan University, Shanghai, 200433 China
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorXiaoshan Zhang
Academy for Engineering and Technology, Institute of Future Lighting, Fudan University, Shanghai, 200433 China
Search for more papers by this authorYikun Wang
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorXiang Wu
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorFeilong Wang
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorQiongrong Ou
Academy for Engineering and Technology, Institute of Future Lighting, Fudan University, Shanghai, 200433 China
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
Search for more papers by this authorCorresponding Author
Shuyu Zhang
Academy for Engineering and Technology, Institute of Future Lighting, Fudan University, Shanghai, 200433 China
School of Information Science and Technology, Institute for Electric Light Sources, Fudan University, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this author† Dedicated to the Special Issue of Perovskite.
Abstract
Comprehensive Summary
Rare earth (RE) ions, with abundant 4f energy level and unique electronic arrangement, are considered as substitutes for Pb2+ in perovskite nanocrystals (PNCs), allowing for partial or complete replacement of lead and minimizing environmental impact. This review provides a comprehensive overview of the characteristics of RE-doped PNCs, including up-conversion luminescence, down-conversion luminescence, and quantum confinement effects, etc. Additionally, RE doping has been found to effectively suppress defect formation, reduce nonradiative recombination, enhance photoluminescence quantum yield (PLQY), and even allow for controlling over the morphology of the nanocrystals. The review also highlights the recent advancements in lead-free RE-based perovskites, especially in the case of Eu-based perovskites (CsEuBr3 and CsEuCl3). Furthermore, it briefly introduces the applications of PNCs in various fields, such as perovskite solar cells (PSCs), luminescent solar concentrators (LSCs), photodetectors (PDs), and light-emitting diodes (LEDs). A systematic discussion on the luminescence mechanisms of RE-doped PNCs and lead-free RE-based perovskites is provided, along with an outlook on future research directions. The ultimate goal of this review is to provide guidance for the development of RE-based perovskite optoelectronic devices.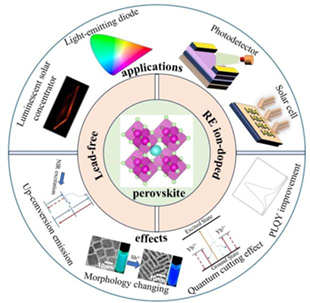
Key Scientists
In 2015, Kovalenko et al. pioneered the synthesis of lead-based perovskite nanocrystals using the thermal injection method. Concurrently, Zhong et al. introduced the ligand-assisted reprecipitation method. These methods have become the predominant approaches for fabricating lead-based perovskite nanocrystals. In 2017, Song et al. successfully incorporated various rare earth ions (Ce3+, Sm3+, Eu3+, Tb3+, Dy3+, Er3+, and Yb3+) into CsPbCl3 perovskite nanocrystals. They also observed the quantum cutting effect induced by defect states, facilitated by the doping of Yb3+. Gamelin et al. subsequently proposed that CsPbCl3:Yb3+ nanocrystals exhibit quantum cutting effects due to the introduction of charge-compensating defects (VPb), resulting in the formation of Yb3+-VPb-Yb3+ defect complexes with shallow defect levels. In 2020, Zhang et al. successfully doped Nd3+ into CsPbBr3, yielding blue luminescent nanocrystals with a central wavelength of 459 nm and up to 90% photo-luminescence quantum yield (PLQY). In the same year, Yang et al. achieved the synthesis of pure phase CsEuCl3 perovskite nanocrystals for the first time. In 2022, Paik et al. synthesized Cs3LnCl6 (Ln = Y, Ce, Gd, Er, Tm, Yb, Eu, Tb) nanocrystals using the thermal injection method. In 2023, Zhang et al. successfully introduced Ni2+ doping into CsEuCl3, enhancing the PLQY of CsEuCl3 nanocrystals from 5% to 19.7%. This review focuses on the development history of perovskite nanocrystals, including rare earth-doped lead-based perovskite nanocrystals and rare earth-based lead-free perovskite nanocrystals, as well as their applications.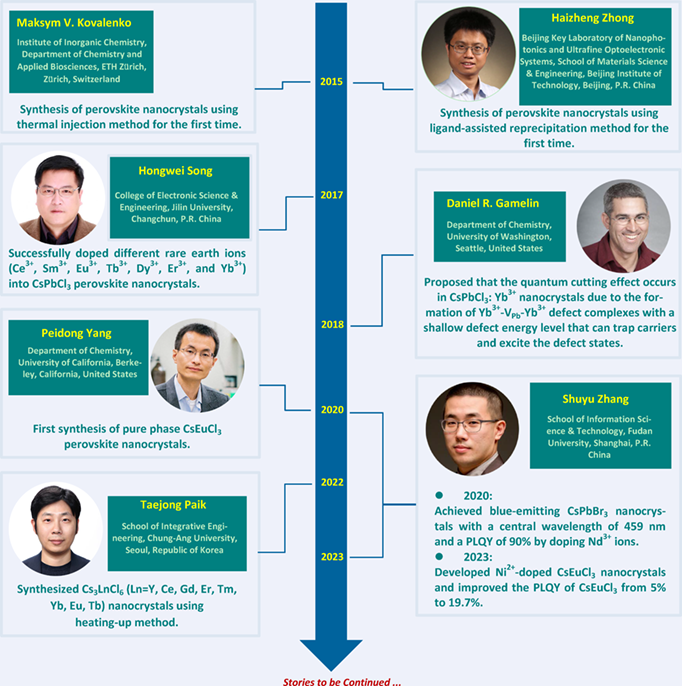
References
- 1 Shamsi, J.; Urban, A. S.; Imran, M. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348.
- 2 Wei, Y.; Cheng, Z. Y.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350.
- 3 Zhu, H. Y.; Zhang, P. F.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385.
- 4 Yin, W. J.; Weng, B. C.; Ge, J. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019, 12, 442–462.
- 5 Cliffe, M. J.; Keyzer, E. N.; Dunstan, M. T. Strongly coloured thiocyanate frameworks with perovskite-analogue structures. Chem. Sci. 2019, 10, 793–801.
- 6 Jiang, Y.; Leyden, M. R.; Qiu, L. B. Combination of Hybrid CVD and Cation Exchange for Upscaling Cs-Substituted Mixed Cation Perovskite Solar Cells with High Efficiency and Stability. Adv. Funct. Mater. 2018, 28, 1703835.
- 7 Saliba, M.; Matsui, T.; Seo, J. Y. Cesium-containing triple cation perovskite solar cells: improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997.
- 8 Jeon, N. J.; Noh, J. H.; Yang, W. S. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480.
- 9 Yao, J. S.; Ge, J.; Han, B. N. Ce3+-Doping to Modulate Photoluminescence Kinetics for Efficient CsPbBr3 Nanocrystals Based Light- Emitting Diodes. J. Am. Chem. Soc. 2018, 140, 3626–3634.
- 10 Miao, J. L.; Zhang, F. J. Recent progress on highly sensitive perovskite photodetectors. J. Mater. Chem. C 2019, 7, 1741–1791.
- 11 Dou, L. T.; Yang, Y.; You, J. B. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404.
- 12 Dastidar, S.; Hawley, C. J.; Dillon, A. D. Quantitative Phase-Change Thermodynamics and Metastability of Perovskite-Phase Cesium Lead Iodide. J. Phys. Chem. Lett. 2017, 8, 1278–1282.
- 13 Han, Q. F.; Bae, S. H.; Sun, P. Y. Single Crystal Formamidinium Lead Iodide (FAPbI3): Insight into the Structural, Optical, and Electrical Properties. Adv. Mater. 2016, 28, 2253–2258.
- 14 Boyd, C. C.; Cheacharoen, R.; Leijtens, T. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451.
- 15 Xie, Y.; Zhou, A.; Zhang, X. Metal cation substitution of halide perovskite nanocrystals. Nano Res. 2022, 15, 6522–6550.
- 16 Fang, D.; Tong, Y.; Xu, F. T. Preparation of CsSnBr3 perovskite film and its all-inorganic solar cells with planar heterojunction. J. Solid State Chem. 2021, 294, 121902.
- 17 Almutlaq, J.; Mir, W. J.; Gutierrez-Arzaluz, L. CsMnBr3: Lead-Free Nanocrystals with High Photoluminescence Quantum Yield and Picosecond Radiative Lifetime. ACS Mater. Lett. 2021, 3, 290–297.
- 18 Huang, J. M.; Lei, T.; Siron, M. Lead-free Cesium Europium Halide Perovskite Nanocrystals. Nano Lett. 2020, 20, 3734–3739.
- 19 Marin, R.; Jaque, D. Doping Lanthanide Ions in Colloidal Semiconductor Nanocrystals for Brighter Photoluminescence. Chem. Rev. 2021, 121, 1425–1462.
- 20 Li, N. X.; Tao, S. X.; Chen, Y. H. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat. Energy 2019, 4, 408–415.
- 21 Wang, L. G.; Zhou, H. P.; Hu, J. N. A Eu3+-Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270.
- 22 Wen, S. H.; Zhou, J. J.; Zheng, K. Z. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415.
- 23 Chen, G. Y.; Qiu, H. L.; Prasad, P. N. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214.
- 24 Woodruff, D. N.; Winpenny, R. E. P.; Layfield, R. A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148.
- 25 Marin, R.; Brunet, G.; Murugesu, M. Shining New Light on Multifunctional Lanthanide Single-Molecule Magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746.
- 26 Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520.
- 27 Zheng, B. Z.; Fan, J. Y.; Chen, B. Rare-Earth Doping in Nanostructured Inorgansic Materials. Chem. Rev. 2022, 122, 5519–5603.
- 28
Sun, R.; Zhou, D.; Song, H. Rare earth doping in perovskite luminescent nanocrystals and photoelectric devices. Nano Select. 2021, 3, 531–554.
10.1002/nano.202100187 Google Scholar
- 29 Mir, W. J.; Sheikh, T.; Arfin, H. Lanthanide doping in metal halide perovskite nanocrystals: spectral shifting, quantum cutting and optoelectronic applications. NPG Asia Mater. 2020, 12, 531–554.
- 30 Protesescu, L.; Yakunin, S.; Bodnarchuk, M. I. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696.
- 31 Zhang, F. Y.; Yang, B.; Zheng, K. B. Formamidinium Lead Bromide (FAPbBr3) Perovskite Microcrystals for Sensitive and Fast Photodetectors. Nano-Micro Lett. 2018, 10, 43.
- 32 Stoumpos, C. C.; Kanatzidis, M. G. The Renaissance of Halide Perovskites and Their Evolution as Emerging Semiconductors. ACS Appl. Mater. Interfaces 2015, 48, 2791–2802.
- 33 Stoumpos, C. C.; Malliakas, C. D.; Kanatzidis, M. G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038.
- 34 Kieslich, G.; Sun, S. J.; Cheetham, A. K. An extended Tolerance Factor Approach for Organic-Inorganic Perovskites. Chem. Sci. 2015, 6, 3430–3433.
- 35 Li, C. H.; Lu, X. G.; Ding, W. Z. Formability of ABX3 (X = F, Cl, Br, I) halide perovskites. Acta Crystallogr. B 2008, 64, 702–707.
- 36 Filip, M. R.; Giustino, F. The geometric blueprint of perovskites. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 5397–5402.
- 37 Liu, X. H.; Yu, D. J.; Song, X. F. Metal Halide Perovskites: Synthesis, Ion Migration, and Application in Field-Effect Transistors. Small 2018, 14, e1801460.
- 38 Sun, Q. D.; Yin, W. J. Thermodynamic Stability Trend of Cubic Perovskites. J. Am. Chem. Soc. 2017, 139, 14905–14908.
- 39 Bartel, C. J.; Sutton, C.; Goldsmith, B. R. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693.
- 40 Umebayashi, T.; Asai, K.; Kondo, T. Electronic structures of lead iodide based low-dimensional crystals. Phys. Rev. B 2003, 67, 155405.
- 41 Butler, K. T.; Frost, J. M.; Walsh, A. Band alignment of the hybrid halide perovskites CH3NH3PbCl3, CH3NH3PbBr3 and CH3NH3PbI3. Mater. Horizons 2015, 2, 228–231.
- 42 Dey, A.; Ye, J. Z.; De, A. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775–10981.
- 43 Xiao, Z. W.; Song, Z. N.; Yan, Y. F. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, e1803792.
- 44 Wang, B. H.; Xiao, X. D.; Chen, T. Perovskite photovoltaics: a high-efficiency newcomer to the solar cell family. Nanoscale 2014, 6, 12287–12297.
- 45 Mosconi, E.; Amat, A.; Nazeeruddin, M. K. First-Principles Modeling of Mixed Halide Organometal Perovskites for Photovoltaic Applications. J. Phys. Chem. C 2013, 117, 13902–13913.
- 46 Ravi, V. K.; Markad, G. B.; Nag, A. Band Edge Energies and Excitonic Transition Probabilities of Colloidal CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals. ACS Energy Lett. 2016, 1, 665–671.
- 47 Yin, W. J.; Shi, T. T.; Yan, Y. F. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 2014, 104, 063903.
- 48 Buin, A.; Comin, R.; Xu, J. X. Halide-Dependent Electronic Structure of Organolead Perovskite Materials. Chem. Mater. 2015, 27, 4405–4412.
- 49 Kang, J.; Wang, L. W. High Defect Tolerance in Lead Halide Perovskite CsPbBr3. J. Phys. Chem. Lett. 2017, 8, 489–493.
- 50 Shi, H. L.; Du, M. H. Shallow halogen vacancies in halide optoelectronic materials. Phys. Rev. B 2014, 90, 174103.
- 51 Brandt, R. E.; Stevanovic, V.; Ginley, D. S. Identifying defect-tolerant semiconductors with high minority-carrier lifetimes: beyond hybrid lead halide perovskites. MRS Commun. 2015, 5, 265–275.
- 52 Hund, F. Concerning the interpretation of complex spectra, especially the elements scandium to nickel. Z. Phys. 1925, 33, 345–371.
- 53 Onodera, Y.; Toyozawa, Y. Excitons in Alkali Halides. J. Phys. Soc. Jpn. 1967, 22, 833–844.
- 54 Becker, M. A.; Vaxenburg, R.; Nedelcu, G. Bright triplet excitons in caesium lead halide perovskites. Nature 2018, 553, 189–193.
- 55 Akkerman, Q. A.; Raino, G.; Kovalenko, M. V. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 2018, 17, 394–405.
- 56 Isarov, M.; Tan, L. Z.; Bodnarchuk, M. I. Rashba Effect in a Single Colloidal CsPbBr3 Perovskite Nanocrystal Detected by Magneto-Optical Measurements. Nano Lett. 2017, 17, 5020–5026.
- 57 Sercel, P. C.; Lyons, J. L.; Wickramaratne, D. Exciton Fine Structure in Perovskite Nanocrystals. Nano Lett. 2019, 19, 4068–4077.
- 58 Choi, Y. J.; Debbichi, L.; Lee, D. K. Light Emission Enhancement by Tuning the Structural Phase of APbBr3 (A = CH3NH3, Cs) Perovskites. J. Phys. Chem. Lett. 2019, 10, 2135–2142.
- 59 Liu, X. K.; Xu, W.; Bai, S. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21.
- 60 Zhang, F.; Zhong, H. Z.; Chen, C. Brightly Luminescent and Color- Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542.
- 61 Li, X. M.; Wu, Y.; Zhang, S. L. CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445.
- 62 Protesescu, L.; Yakunin, S.; Nazarenko, O. Low-Cost Synthesis of Highly Luminescent Colloidal Lead Halide Perovskite Nanocrystals by Wet Ball Milling. ACS Appl. Nano Mater. 2018, 1, 1300–1308.
- 63 Pan, Q.; Hu, H. C.; Zou, Y. T. Microwave-assisted synthesis of high- quality "all-inorganic” CsPbX3 (X = Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes. J. Mater. Chem. C 2017, 5, 10947–10954.
- 64 Chouhan, L.; Ghimire, S.; Subrahmanyam, C. Synthesis, optoelectronic properties and applications of halide perovskites. Chem. Soc. Rev. 2020, 49, 2869–2885.
- 65 Yang, Z. Y.; Peng, S. M.; Lin, F. Self-assembly Behavior of Metal Halide Perovskite Nanocrystals. Chin. J. Chem. 2022, 40, 2239–2248.
- 66 Bunzli, J. C. G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47.
- 67 Qin, X.; Liu, X. W.; Huang, W. Lanthanide-Activated Phosphors Based on 4f-5d Optical Transitions: Theoretical and Experimental Aspects. Chem. Rev. 2017, 117, 4488–4527.
- 68 Duan, C. K.; Tanner, P. A.; Meijerink, A. 4f-5d Transitions of Tb3+ in Cs2NaYF6: The Effect of Distortion of the Excited-State Configuration. J. Phys. Chem. A 2011, 115, 9188–9191.
- 69 Zhao, H. Y.; Xia, J. L.; Yin, D. D. Rare earth incorporated electrode materials for advanced energy storage. Coord. Chem. Rev. 2019, 390, 32–49.
- 70 Xu, J.; Chen, X. Y.; Xu, Y. S. Ultrathin 2D Rare-Earth Nanomaterials: Compositions, Syntheses, and Applications. Adv. Mater. 2020, 32, e1806461.
- 71 Martin-Rodriguez, R.; Geitenbeek, R.; Meijerink, A. Incorporation and Luminescence of Yb3+ in CdSe Nanocrystals. J. Am. Chem. Soc. 2013, 135, 13668–13671.
- 72 Creutz, S. E.; Fainblat, R.; Kim, Y. A Selective Cation Exchange Strategy for the Synthesis of Colloidal Yb3+-Doped Chalcogenide Nanocrystals with Strong Broadband Visible Absorption and Long-Lived Near-Infrared Emission. J. Am. Chem. Soc. 2017, 139, 11814–11824.
- 73 Xiang, W. C.; Wang, Z. W.; Kubicki, D. J. Europium-Doped CsPbI2Br for Stable and Highly Efficient Inorganic Perovskite Solar Cells. Joule 2019, 3, 205–214.
- 74 Wu, X. W.; Li, H. W.; Wang, K. CH3NH3Pb1-xEuxI3 mixed halide perovskite for hybrid solar cells: the impact of divalent europium doping on efficiency and stability. RSC Adv. 2018, 8, 11095–11101.
- 75 Pan, G. C.; Bai, X.; Yang, D. W. Doping Lanthanide into Perovskite Nanocrystals: Highly Improved and Expanded Optical Properties. Nano Lett. 2017, 17, 8005–8011.
- 76 Cai, T.; Wang, J. Y.; Li, W. H. Mn2+/Yb3+ Codoped CsPbCl3 Perovskite Nanocrystals with Triple-Wavelength Emission for Luminescent Solar Concentrators. Adv. Sci. 2020, 7, 2001317.
- 77 Bloembergen, N. Solid State Infrared Quantum Counters. Phys. Rev. Lett. 1959, 2, 84–85.
- 78 Zeng, M.; Singh, S.; Hens, Z. Strong upconversion emission in CsPbBr3 perovskite quantum dots through efficient BaYF5:Yb,Ln sensitization. J. Mater. Chem. C 2019, 7, 2014–2021.
- 79 Frances-Soriano, L.; Gonzalez-Carrero, S.; Navarro-Raga, E. Efficient Cementing of CH3NH3PbBr3 Nanoparticles to Upconversion Nanoparticles Visualized by Confocal Microscopy. Adv. Funct. Mater. 2016, 26, 5131–5138.
- 80 Zheng, W.; Huang, P.; Gong, Z. L. Near-infrared-triggered photon upconversion tuning in all-inorganic cesium lead halide perovskite quantum dots. Nat. Commun. 2018, 9, 3462.
- 81 Marin, R.; Labrador-Paez, L.; Skripka, A. Upconverting Nanoparticle to Quantum Dot Forster Resonance Energy Transfer: Increasing the Efficiency through Donor Design. ACS Photonics 2018, 5, 2261–2270.
- 82 Stryer, L.; Haugland, R. P. Energy Transfer: A Spectroscopic Ruler. Proc. Natl. Acad. Sci. U. S. A. 1967, 58, 719–726.
- 83 He, L. J.; Meng, J. L.; Feng, J. Unveiling the mechanism of rare earth doping to optimize the optical performance of the CsPbBr3 perovskite. Inorg. Chem. Front. 2020, 7, 4669–4676.
- 84 Liu, W. Y.; Lin, Q. L.; Li, H. B. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961.
- 85 Parobek, D.; Roman, B. J.; Dong, Y. T. Exciton-to-Dopant Energy Transfer in Mn-Doped Cesium Lead Halide Perovskite Nanocrystals. Nano Lett. 2016, 16, 7376–7380.
- 86 Liu, H. W.; Wu, Z. N.; Shao, J. R. CsPbxMn1-xCl3 Perovskite Quantum Dots with High Mn Substitution Ratio. ACS Nano 2017, 11, 2239–2247.
- 87 Hu, Q. S.; Li, Z.; Tan, Z. F. Rare Earth Ion-Doped CsPbBr3 Nanocrystals. Adv. Opt. Mater. 2018, 6, 1700864.
- 88 Jin, S. L.; Li, R. F.; Huang, H. Compact ultrabroadband light-emitting diodes based on lanthanide-doped lead-free double perovskites. Light: Sci. Appl. 2022, 11, 52.
- 89 Cao, L. Y.; Jia, X. F.; Gan, W. J. Strong Self-Trapped Exciton Emission and Highly Efficient Near-Infrared luminescence in Sb3+-Yb3+ Co-doped Cs2AgInCl6 Double Perovskite. Adv. Funct. Mater. 2023, 33, 2212135.
- 90 Dexter, D. L. Possibility of Luminescent Quantum Yields Greater than Unity. Phys. Rev. 1957, 108, 630–633.
- 91 Li, X. Y.; Duan, S.; Liu, H. C. Mechanism for the Extremely Efficient Sensitization of Yb3+ Luminescence in CsPbCl3 Nanocrystals. J. Phys. Chem. Lett. 2019, 10, 487–492.
- 92 Milstein, T. J.; Kroupa, D. M.; Gamelin, D. R. Picosecond Quantum Cutting Generates Photoluminescence Quantum Yields Over 100% in Ytterbium-Doped CsPbCl3 Nanocrystals. Nano Lett. 2018, 18, 3792–3799.
- 93 Milstein, T. J.; Kluherz, K. T.; Kroupa, D. M. Anion Exchange and the Quantum-Cutting Energy Threshold in Ytterbium-Doped CsPb(Cl1-xBrx)3 Perovskite Nanocrystals. Nano Lett. 2019, 19, 1931–1937.
- 94 Zhou, D. L.; Liu, D. L.; Pan, G. C. Cerium and Ytterbium Codoped Halide Perovskite Quantum Dots: A Novel and Efficient Down-converter for Improving the Performance of Silicon Solar Cells. Adv. Mater. 2017, 29, 1704149.
- 95 Zhou, D. L.; Sun, R.; Xu, W. Impact of Host Composition, Codoping, or Tridoping on Quantum-Cutting Emission of Ytterbium in Halide Perovskite Quantum Dots and Solar Cell Applications. Nano Lett. 2019, 19, 6904–6913.
- 96 Xie, Y. J.; Peng, B.; Bravic, I. Highly Efficient Blue-Emitting CsPbBr3 Perovskite Nanocrystals through Neodymium Doping. Adv. Sci. 2020, 15, 6522–6550.
- 97 Das, S.; De, A.; Samanta, A. Ambient Condition Mg2+ Doping Producing Highly Luminescent Green- and Violet-Emitting Perovskite Nanocrystals with Reduced Toxicity and Enhanced Stability. J. Phys. Chem. Lett. 2020, 11, 1178–1188.
- 98 Bi, C. H.; Wang, S. X.; Li, Q. Thermally Stable Copper(II)-Doped Cesium Lead Halide Perovskite Quantum Dots with Strong Blue Emission. J. Phys. Chem. Lett. 2019, 10, 943–952.
- 99 Lv, Q. R.; Li, C.; Zhang, L. J. Tailoring Photon Emission from CH3NH3PbBr3 Quantum Dots through Mn-Substitution. J. Phys. Chem. C 2021, 125, 14311–14316.
- 100 Liu, N.; Yam, C. Y. First-principles study of intrinsic defects in formamidinium lead triiodide perovskite solar cell absorbers. Phys. Chem. Chem. Phys. 2018, 20, 6800–6804.
- 101 Li, W.; Liu, J.; Bai, F. Q. Hole Trapping by Iodine Interstitial Defects Decreases Free Carrier Losses in Perovskite Solar Cells: A Time-Domain ab initio Study. ACS Energy Lett. 2017, 2, 1270–1278.
- 102 Lee, W.; Oh, J.; Kwon, W. Synthesis of Ag/Mn Co-Doped CdS/ZnS (Core/Shell) Nanocrystals with Controlled Dopant Concentration and Spatial Distribution and the Dynamics of Excitons and Energy Transfer between Co-Dopants. Nano Lett. 2019, 19, 308–317.
- 103 Lee, W.; Hong, S.; Kim, S. Colloidal Synthesis of Lead-Free Silver Indium Double-Perovskite Cs2AgInCl6 Nanocrystals and Their Doping with Lanthanide Ions. J. Phys. Chem. C 2019, 123, 2665–2672.
- 104 Pan, G. C.; Bai, X.; Xu, W. Impurity Ions Codoped Cesium Lead Halide Perovskite Nanocrystals with Bright White Light Emission toward Ultraviolet-White Light-Emitting Diode. ACS Appl. Mater. Interfaces 2018, 10, 39040–39048.
- 105 Chen, N.; Cai, T.; Li, W. H. Yb- and Mn-Doped Lead-Free Double Perovskite Cs2AgBiX6 (X = Cl-, Br-) Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 16855–16863.
- 106 Ahmed, G. H.; El-Demellawi, J. K.; Yin, J. Giant Photoluminescence Enhancement in CsPbCl3 Perovskite Nanocrystals by Simultaneous Dual-Surface Passivation. ACS Energy Lett. 2018, 3, 2301–2307.
- 107 Zhai, Y.; Bai, X.; Pan, G. C. Effective blue-violet photoluminescence through lanthanum and fluorine ions co-doping for CsPbCl3 perovskite quantum dots. Nanoscale 2019, 11, 2484–2491.
- 108 Luo, C.; Li, W.; Xiong, D. Surface pre-optimization of a mixed halide perovskite toward high photoluminescence quantum yield in the blue spectrum range. Nanoscale 2019, 11, 15206–15215.
- 109 Chiba, T.; Sato, J.; Ishikawa, S. Neodymium Chloride-Doped Perovskite Nanocrystals for Efficient Blue Light-Emitting Devices. ACS Appl. Mater. Interfaces 2020, 12, 53891–53898.
- 110 Zhou, A. Q.; Xie, Y. J.; Wang, F. L. High-Efficiency Fast-Radiative Blue-Emitting Perovskite Nanoplatelets and Their Formation Mechanisms. J. Phys. Chem. Lett. 2022, 13, 4634–4641.
- 111 Shen, Z. H.; Qiao, B.; Xu, Z. The luminescence properties of CsPbxM1-xBr3 perovskite nanocrystals transformed from Cs4PbBr6 mediated by various divalent bromide MBr2 salts. Nanoscale 2019, 11, 4008–4014.
- 112 Akkerman, Q. A.; Park, S.; Radicchi, E. Nearly Monodisperse Insulator Cs4PbX6 (X = Cl, Br, I) Nanocrystals, Their Mixed Halide Compositions, and Their Transformation into CsPbX3 Nanocrystals. Nano Lett. 2017, 17, 1924–1930.
- 113 Liu, Z. K.; Bekenstein, Y.; Ye, X. C. Ligand Mediated Transformation of Cesium Lead Bromide Perovskite Nanocrystals to Lead Depleted Cs4PbBr6 Nanocrystals. J. Am. Chem. Soc. 2017, 139, 5309–5312.
- 114 Luo, J. J.; Yang, L. B.; Tan, Z. F. Efficient Blue Light Emitting Diodes Based On Europium Halide Perovskites. Adv. Mater. 2021, 33, 2101903.
- 115 Wang, L.; Guo, Q. X.; Duan, J. S. Exploration of Nontoxic Cs3CeBr6 for Violet Light-Emitting Diodes. ACS Energy Lett. 2021, 6, 4245–4254.
- 116 Moon, B.; Kim, S. J.; Lee, S. Rare-Earth-Element-Ytterbium-Substituted Lead-Free Inorganic Perovskite Nanocrystals for Optoelectronic Applications. Adv. Mater. 2019, 31, e1901716.
- 117 Li, J. Y.; Wang, L. D.; Zhao, Z. F. Highly efficient and air-stable Eu(II)-containing azacryptates ready for organic light-emitting diodes. Nat. Commun. 2020, 11, 5218.
- 118 Walsh, K. M.; Pressler, K.; Crane, M. J. Ferromagnetism and Spin-Polarized Luminescence in Lead-Free CsEuCl3 Perovskite Nanocrystals and Thin Films. ACS Nano 2022, 16, 2569–2576.
- 119 Straus, D. B.; Klimczuk, T.; Xu, X. H. Antiferromagnetic Order in the Rare-Earth Halide Perovskites CsEuBr3 and CsEuCl3. Chem. Mater. 2022, 34, 10772–10777.
- 120 Gao, Y. L.; Zhang, T.; Liu, J. Enhanced photoluminescence stability and internal defect evolution of the all-inorganic lead-free CsEuCl3 perovskite nanocrystals. Phys. Chem. Chem. Phys. 2022, 24, 18860–18867.
- 121 Zhang, X. S.; Wang, F. L.; Wang, Y. K. Boosting the Photoluminescence Quantum Yield and Stability of Lead-Free CsEuCl3 Nanocrystals via Ni2+ Doping. J. Phys. Chem. Lett. 2023, 14, 5580–5585.
- 122 Fu, X. Y.; Li, Y.; Li, H. W. Cerium Ion Promoting the Emission of Self-Trapped Excitons of Quasi-2D Europium Halide Perovskites for Self-Activated White Light-Emitting. J. Phys. Chem. C 2023, 127, 8159–8166.
- 123 Alam, F.; Wegner, K. D.; Pouget, S. Eu2+: A suitable substituent for Pb2+ in CsPbX3 perovskite nanocrystals? J. Chem. Phys. 2019, 151, 231101.
- 124 Yang, Z. W.; Jiang, Z.; Liu, X. Y. Bright Blue Light-Emitting Doped Cesium Bromide Nanocrystals: Alternatives of Lead-Free Perovskite Nanocrystals for White LEDs. Adv. Opt. Mater. 2019, 7, 1900108.
- 125 Shamsi, J.; Dang, Z. Y.; Ijaz, P. Colloidal CsX (X = CI, Br, I) Nanocrystals and Their Transformation to CsPbX3 Nanocrystals by Cation Exchange. Chem. Mater. 2018, 30, 79–83.
- 126 Lee, M.; Lee, D. H. D.; Hong, S. V. Highly Luminescent and Multifunctional Zero-Dimensional Cesium Lanthanide Chloride (Cs3LnCl6) Colloidal Nanocrystals. Adv. Opt. Mater. 2022, 10, 2102727.
- 127 Lee, M.; Chung, H.; Hong, S. V. Dynamically tunable multicolor emissions from zero-dimensional Cs3LnCl6 (Ln: europium and terbium) nanocrystals with wide color gamut. Nanoscale 2023, 15, 1513–1521.
- 128 Chen, X.; Xu, W.; Song, H. W. Highly Efficient LiYF4:Yb3+, Er3+ Upconversion Single Crystal under Solar Cell Spectrum Excitation and Photovoltaic Application. ACS Appl. Mater. Interfaces 2016, 8, 9071–9079.
- 129 Zhang, Y. D.; Xiao, S. Q.; Sheng, W. P. Improving Ultraviolet Stability of Perovskite Solar Cells via Singlet Fission Down-Conversion. Chin. J. Chem. 2023, 41, 1057–1065.
- 130 Luo, X.; Ding, T.; Liu, X. Quantum-Cutting Luminescent Solar Concentrators Using Ytterbium-Doped Perovskite Nanocrystals. Nano Lett. 2019, 19, 338–341.
- 131
Wei, T.; Lian, K.; Tao, J. Q. Mn-Doped Multiple Quantum Well Perovskites for Efficient Large-Area Luminescent Solar Concentrators. ACS Appl. Mater. Interfaces 2022, 15, 44572–44580.
10.1021/acsami.2c12834 Google Scholar
- 132 Crane, M. J.; Kroupa, D. M.; Gamelin, D. R. Detailed-balance analysis of Yb3+:CsPb(Cl1-xBrx)3 quantum-cutting layers for high-efficiency photovoltaics under real-world conditions. Energy Environ. Sci. 2019, 12, 2486–2495.
- 133 Ding, N.; Xu, W.; Zhou, D. L. Upconversion ladder enabled super- sensitive narrowband near-infrared photodetectors based on rare earth doped florine perovskite nanocrystals. Nano Energy 2020, 76, 105103.
- 134 Ding, N.; Xu, W.; Zhou, D. L. Extremely efficient quantum-cutting Cr3+, Ce3+, Yb3+ tridoped perovskite quantum dots for highly enhancing the ultraviolet response of Silicon photodetectors with external quantum efficiency exceeding 70%. Nano Energy 2020, 78, 105278.
- 135 Cheng, Y. Z.; Shen, C. Y.; Shen, L. L. Tb3+, Eu3+ Co-doped CsPbBr3 QDs Glass with Highly Stable and Luminous Adjustable for White LEDs. ACS Appl. Mater. Interfaces 2018, 10, 21434–21444.
- 136 Sun, R.; Lu, P.; Zhou, D. L. Samarium-Doped Metal Halide Perovskite Nanocrystals for Single-Component Electroluminescent White Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 2131–2139.
- 137 Gonzalez-Carrero, S.; Galian, R. E.; Pérez-Prieto, J. Maximizing the emissive properties of CH3NH3PbBr3 perovskite nanoparticles. J. Mater. Chem. A 2015, 3, 9187–9193.
- 138 Yong, Z. J.; Guo, S. Q.; Ma, J. P. Doping-Enhanced Short-Range Order of Perovskite Nanocrystals for Near-Unity Violet Luminescence Quantum Yield. J. Am. Chem. Soc. 2018, 140, 9942–9951.
- 139 Yang, J. N.; Song, Y.; Yao, J. S. Potassium Bromide Surface Passivation on CsPbI(3-x)Br(x) Nanocrystals for Efficient and Stable Pure Red Perovskite Light-Emitting Diodes. J Am Chem Soc. 2020, 142, 2956–2967.
- 140 Krieg, F.; Ong, Q. K.; Burian, M. Stable Ultraconcentrated and Ultradilute Colloids of CsPbX(3) (X = Cl, Br) Nanocrystals Using Natural Lecithin as a Capping Ligand. J. Am. Chem. Soc. 2019, 141, 19839–19849.
- 141 Krieg, F.; Ochsenbein, S. T.; Yakunin, S. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646.




