Long-term renal survival of γ3-heavy-chain deposition disease complicated by heart failure: A case report
Shujun Shi and Kaiying He contributed equally to this article.
Abstract
Key Clinical Message
Heavy-chain deposition disease (HCDD), a rare monoclonal immunoglobulin deposition disease, involves truncated heavy-chain deposition in kidneys. Limited long-term data exist. We report a case of renal and cardiac failure with favorable outcomes post bortezomib-based therapy. Stable renal function observed over 4 years suggests efficacy in HCDD with multisystem involvement.
Heavy-chain deposition disease (HCDD) is an extremely rare form of monoclonal immunoglobulin deposition disease (MIDD) that involves the deposition of truncated immunoglobulin heavy chains in the kidneys. Only a few cases of HCDD with a favorable long-term renal prognosis have been reported, resulting in limited long-term follow-up data for this patient population. In this report, we present the case of a 52-year-old patient with nephrotic syndrome who experienced renal failure and cardiac failure. Renal biopsy confirmed the presence of γ3-HCDD and monoclonal Immunoglobulin G (IgG)κ in the serum. The patient exhibited low voltage on electrocardiogram (ECG) and unexplained left ventricular hypertrophy on cardiac ultrasound. The patient underwent eight cycles of bortezomib-based chemotherapy, which led to hematological remission. After 4 years of follow-up, the patient's renal function remained stable, with serum creatinine levels ranging from 0.7 to 0.9 mg/dL and proteinuria of 0.3–0.5 g/24 h. Our findings suggest that bortezomib-based chemotherapy is equally effective in HCDD patients with combined multisystem damage.
1 INTRODUCTION
Heavy-chain deposition disease (HCDD) is an exceptionally rare form of monoclonal immunoglobulin deposition disease (MIDD) found in less than 1% of native kidney biopsies. It is characterized by the presence of truncated tissue deposits of monoclonal immunoglobulin heavy chains in various organs and tissues, leading to progressive dysfunction and damage.1-4 The global reported cases of HCDD are fewer than 80,4, 5 and its rarity limits the available epidemiological information. HCDD predominantly affects adults, with a slightly higher incidence in males compared to females. While the disease can manifest at any age, it commonly arises between the ages of 40 and 60. The primary clinical presentations of HCDD include proteinuria, hematuria, and renal failure, which can advance to end-stage renal disease. Additionally, hepatomegaly, splenomegaly, and lymphadenopathy are frequently observed.2, 4 Less frequently, peripheral neuropathy and cardiac disease may also manifest.3, 6
The precise etiology of HCDD remains incompletely understood; however, it is believed to be associated with abnormalities in the synthesis or processing of immunoglobulin heavy chains. These aberrant heavy chains are released into the bloodstream and deposited in the kidneys, inciting an inflammatory response and causing damage to renal tissue.7-10 Diagnosis primarily relies on the pathological findings from a renal biopsy. The therapeutic approach for HCDD often involves addressing the underlying B-cell disorder. Unfortunately, HCDD has exhibited unfavorable outcomes in many cases, with patients experiencing a median renal survival of only 28 months, even when treated with immunosuppressive agents.11 Patel et al.6 and small case series have reported that bortezomib can induce hematological responses and improve renal function in HCDD patients.12-14 The mechanism by which bortezomib operates in HCDD is not yet fully comprehended, but it is believed to be associated with inhibiting plasma cell proliferation and reducing the levels of circulating monoclonal immunoglobulins. In this report, we present a case of HCDD with renal and hematological involvement along with heart failure, successfully treated with bortezomib-based chemotherapy. The patient has been under observation for 57 months, demonstrating long-term preservation of kidney function, which contributes to our understanding of the disease.
Written informed consent was obtained from the patient for the publication of this report.
2 CASE HISTORY
A 52-year-old female patient was admitted with peripheral edema, heavy proteinuria, and worsening dyspnea. A previous renal biopsy had revealed nodular glomerulosclerosis, but no further characterization of immunofluorescence features was performed at that time. Initially, the patient was treated with valsartan, an angiotensin II receptor blocker, for nodular sclerosing glomerulopathy (NGS), but showed no improvement after 3 months. Additionally, she experienced progressive dyspnea and an increase in the serum creatinine levels. Over time, the serum creatinine level rose from 0.8 to 1.7 mg/dL (normal range: 0.50–1.2 mg/dL), while urinary protein excretion increased from 1.51 to 2.19 g/24 h (normal range: <0.15 g/24 h). Due to these worsening symptoms, she was admitted to our hospital for further treatment. The patient had no history of hypertension or chronic heart disease, and a physical examination a year ago revealed a blood pressure of 127/74 mmHg with no abnormalities detected during cardiac ultrasound. On admission, her blood pressure was measured as 169/97 mmHg, heart rate was 62/min, and body mass index (BMI) was 22.03 kg/m2. Skin examination revealed no signs of jaundice, rash, or bleeding, and there were no palpable superficial lymph nodes. Bilateral pitting edema was observed in the lower limbs.
Detailed methods for further investigations are outlined in Table 1.
| Urinalysis | Blood chemistry | Serology | |||
|---|---|---|---|---|---|
| Protein | 3 + (Negative) | Blood urea nitrogen (mg/dL) | 23.25 (8–21) | C-reactive protein (mg/L) | 0.45 (<10) |
| 24-h urine protein (g) | 4.71 (<0.15) | Creatinine (mg/dL) | 1.92 (0.5–1.5) | Rheumatoid factor (IU/mL) | <20 |
| Red blood cell (cells/high-power field) | 40–60 (0–7) | eGFR (mL/min/1.73 m2)a | 29.51 | Antinuclear antibodies | 1:100 (Negative) |
| RBC cast | 0 (0) | Albumin (g/L) | 27.9 (40–55) | Anti-ENA autoantibodies | Negative |
| White blood cell (cells/high-power field) | 40 (0–12) | Total cholesterol (mg/dL) | 187.9 (90–200) | MPO-ANCA | Negative |
| Urine culture | No growth of bacteria | Aspartate aminotransferase (IU/L) | 17 (15–40) | Anti-GBM | Negative |
| UNAG (U/L) | 10.3 (<12) | Alanine transaminase (IU/L) | 22 (9–50) | PR3-ANCA | Negative |
| β2-Microglobulin (g/L) | 221 (<200) | IgG (g/L) | 10.38 (8.60–17.40) | ||
| Other | Peripheral blood | IgA (g/L) | 1.21 (1.00–4.20) | ||
| Cryoglobulin test | Negative | White blood cell (cells/L) | 5.23 × 109 (3.5–9.5) | IgM (g/L) | 1.85 (0.30–2.20) |
| Tumor marker | Negative | Platelet (cells/L) | 241 × 109 (125–350) | Complement 3 (g/L) | 0.4 (0.70–1.40) |
| Treponema pallidum-specific antibody | Positive (Negative) | Peripheral blood (g/L) | 104 (130–175) | Complement 4 (g/L) | 0.04 (0.10–0.40) |
| T. pallidum particle agglutination test | Negative | Markers of myocardial injury | C1q (mg/L) | 121.6 (160.0–230.0) | |
| Reactive rapid plasma reagin | Negative | NT-proBNP (pg/mL) | 1304 (<125) | ||
| PLA2R(RU/mL) | <5 (<14) | hs-TnT | 16.24 (0–14.00) | ||
| Anti-hepatitis B and C virus antibody | Negative | ||||
| Human immunodeficiency virus | Negative |
- a eGFR, the estimated glomerular filtration rate was calculated using the CKD-EPI equation; PLA2R, anti-phospholipase A2 receptor antibodies; hs-cTnT, high-sensitivity cardiac-specific troponin T; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NAG, N-acetyl-β-D-glucosidase.
The sizes of both kidneys were within the normal range. The electrocardiogram (ECG) indicated a heart rate of 57 bpm, suggesting sinus bradycardia and low voltage criteria in the limb leads. An echocardiogram revealed left ventricular hypertrophy, mild pulmonary hypertension with mild tricuspid valve and aortic regurgitation, left ventricular diastolic dysfunction (E/e 15.32, E/A 1.7), and a left ventricular ejection fraction (EF) of 53%. Additionally, there was thickening of the interatrial septum and a small pericardial effusion.
3 METHODS
3.1 Differential diagnosis
This is a case of a middle-aged woman with a 3-month history of gradually increasing proteinuria and microscopic hematuria, mild elevation of serum creatinine, hypertension, mild anemia, decreased complement levels (C3 and C4), and NGS on kidney biopsy. Based on the clinical presentation of the patient, the differential diagnosis may include the following possibilities.
Membranoproliferative glomerulonephritis (MPGN): The patient exhibits characteristics of MPGN such as significant proteinuria, microscopic hematuria, mild elevation in serum creatinine, and decreased complement levels. However, there is no clear history of infectious diseases, and specific triggers for MPGN are not present.
Lupus nephritis (LN): In addition to the characteristics of MPGN mentioned above, systemic lupus erythematosus can involve the hematologic system and lead to anemia. The patient's tests for ANA and anti-dsDNA antibodies showed negative results, ruling out LN.
Considering the presence of mild anemia in the patient, hematologic disorders should also be considered. Further investigations such as serum light-chain studies, immunofixation electrophoresis, and bone marrow biopsy may be needed to rule out hematologic disorders.
Given the NGS on kidney biopsy and the clinical presentation, the differential diagnosis and analysis are as follows:
Diabetic nephropathy: While it can also manifest as nodular lesions, the absence of abnormal glucose metabolism in the patient's tests makes this diagnosis less likely.
Systemic amyloidosis: Patients with systemic amyloidosis typically experience low blood pressure and concurrent cardiac damage. The absence of PAS-positive homogeneous material deposition and absence of fibril deposits on electron microscopy do not support the diagnosis of amyloidosis.
MIDD: These diseases can present with proteinuria and microscopic hematuria, often accompanied by hypertension. Even though the patient has elevated serum creatinine levels, additional hematologic involvement such as anemia may be present. HCDD is more associated with hypertension, with relatively less extrarenal manifestations, and may be accompanied by low complement levels. Further staining for kidney tissue light and heavy chains is needed for differential diagnosis.
3.2 Investigations
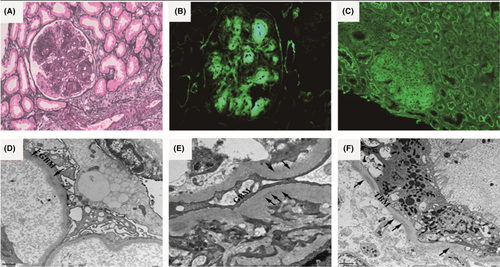
Due to diagnostic uncertainty, a second renal biopsy was performed. Light microscopy examination showed 23 non-sclerotic glomeruli, all of which exhibited proliferation with a diffuse increase in mesangial matrix and mesangial hypercellularity, forming nodular lesions (Figure 1A). Most glomeruli showed lobulation and distinct nodular formations in the mesangial area, which resulted in narrowing of the capillary lumens. Double contour formation of the glomerular basement membrane was observed, while capillary aneurysms were absent. One glomerulus displayed cellular crescents, and the granular basement membrane appeared thickened. Tubular atrophy and multifocal interstitial fibrosis were present, along with mild infiltration of lymphocytes in the interstitium. Mild arteriolar sclerosis and intimal fibrosis were noted in the arteries. Congo red staining yielded negative results. Direct immunofluorescence examination of frozen renal tissue revealed extensive staining (3+) of IgG in the mesangium and along the capillary walls. Staining for IgG was also positive on the tubular basement membrane (TBM). Subclass staining for IgG demonstrated 3+ deposition specifically for IgG3 in the mesangium, along capillary walls, and on tubular basement membranes (Figure 1B). However, staining for IgG1, IgG2, and IgG4 was negative. Linear γ deposits were consistently observed along the TBM, glomerular basement membranes (GBM), and within mesangial nodules, without concurrent light chains (Figure 1C). The glomeruli exhibited granular staining (2+) for C1q and positive staining for C3 in the mesangium and along the capillary walls. All other stains, including IgA, IgM, ALB, and PLA2R, yielded negative results within the glomeruli and throughout the tubulointerstitium. Electron microscopy revealed segmental expansion of the glomerular basement membrane. In the expanded mesangium area, finely punctate-powdery electron-dense deposits were observed in the mesangium, GBM (Figure 1D,E), and TBM (Figure 1F). Effacement of the podocyte foot processes was evident. Based on these pathological findings, the diagnosis in this case corresponded to γ3-HCDD.
A bone marrow biopsy was conducted, which showed normal cellularity and mild plasmacytosis (3%). The Congo red stain yielded negative results. Serum protein immunofixation electrophoresis indicated the presence of a monoclonal IgG κ band. The levels of serum free κ light chain (FLC) were elevated, with a value of 262.5 mg/L (normal range: 3.3–19.4 mg/L), while the λ light-chain level was 74.5 mg/L (normal range: 5.71–26.30 mg/L). The κ/λ ratio was elevated at 3.5235 (normal range: 0.26–1.65). To further explore the relationship between HCDD and abnormal lymphoproliferative disease, an IGH gene rearrangement assay was performed, yielding a positive result for IGH/MAF genes, indicating the presence of plasmacytosis.
3.3 Outcome and follow-up
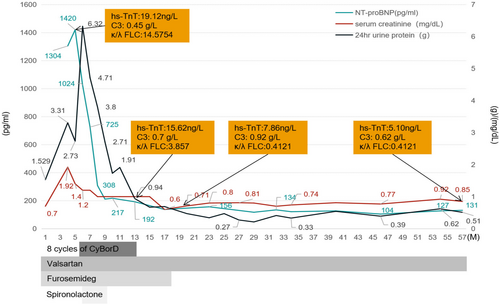
Based on the findings, the patient was diagnosed with HCDD and monoclonal gammopathy of renal significance (MGRS). The treatment and clinical course of the patient are depicted in Figure 2. The patient underwent eight cycles of CyBorD (cyclophosphamide 300 mg/m2/weekly, bortezomib 1.5 mg/m2, and dexamethasone 40 mg/weekly) chemotherapy. The treatment was well-tolerated, and no significant adverse effects such as gastrointestinal reactions, hair loss, or bone marrow suppression were observed. Hematological remission was achieved within 5 months, as indicated by normal serum immunofixation electrophoresis and FLC analysis. The patient has been followed up for a period of 57 months with no recurrence of nephrotic syndrome or heart failure symptoms. The patient's serum creatinine levels ranged from 0.7 to 0.9mg/dL, with a proteinuria range of 0.3–0.5 g/24 h. Blood pressure readings were within the range of 108–138/72–88 mmHg, serum complement C3 levels were within the range of 0.81–1.07 g/L, and the hemoglobin level was between 117 and 122g/L. Repeat echocardiograms showed improved cardiac function, with an EF of 64% and no signs of pulmonary hypertension or pericardial effusion. Other examination results indicated reduced left ventricular hypertrophy, E/e ratio of 11.27, and E/A ratio of 0.96. The CyBorD treatment had a remarkable positive impact on the HCDD in the patient, leading to significant improvements in clinical and laboratory parameters.
4 DISCUSSION
The patient presented with severe proteinuria, microhematuria, hypertension, and rapidly progressive renal failure. She had monoclonal IgGκ gammopathy that did not meet the criteria for multiple myeloma or lymphoma. The histologic findings obtained through routine light microscopy, immunofluorescence, and electron microscopy in this case confirmed the presence of γ3-HCDD, thus establishing the diagnosis of MGRS.
Nodular glomerulosclerosis can manifest as various disease pathologies, including diabetic nephropathy, amyloidosis, and fibrous glomerulonephritis. In this case, the diagnosis of HCDD was based on the absence of Congo red-positive staining in nodular glomerulosclerosis and the identification of IgG heavy-chain deposition without concomitant light-chain staining. Electron microscopy revealed thin strips of electron-dense deposits resembling sediment on the inner side of the segmental GBM and the outer side of the TBM.1, 15 The underlying pathogenesis of nodular glomerulosclerosis in HCDD remains poorly understood. Interestingly, the patient exhibited specific features, such as the deposition of C1q and C3 in the glomeruli and reduced levels of C3 and C1q, which are consistent with previous reports (4). These findings, combined with recent studies, suggest that complement-mediated nodular glomerulosclerosis may play a role in patients with HCDD.16 Activation of the classical and alternative pathways of complement within the kidney, as well as the release of chemotactic complement that recruits leukocytes, may contribute to mesangial matrix expansion. Additionally, the deposited heavy chains may possess exposed groups that activate complement, leading to a mild inflammatory response in the glomerulus and subsequent hematuria development in the patient.17, 18
Although the patient displayed symptoms of heart failure, she had no previous history of chronic heart disease. Her ECG revealed low voltage and unexplained left ventricular hypertrophy. However, aggressive blood pressure control, volume reduction, and correction of anemia did not result in normalization of her NT-proBNP and hs-TNT levels. These findings led us to speculate that her heart failure symptoms were associated with MGRS-related HCDD. We hypothesize that heavy-chain deposition in the myocardium contributes to myocardial degeneration, myocardial hypertrophy, and impaired diastolic and systolic function of the heart. Furthermore, the monoclonal immunoglobulinemia leads to hyperviscosity, tissue stasis, and hypoxic factors, which further exacerbate cardiac injury. To date, only a few cases of HCDD with renal involvement and accompanying skin, thyroid, or liver damage have been reported.1, 2, 4, 8, 19 However, cases involving both cardiac and renal lesions are extremely rare, with only two previous reports of similar patients.3 Unfortunately, the patient did not provide consent for a heart biopsy to further elucidate the cause of the cardiac damage. This finding underscores the importance of comprehensive clinical evaluation in HCDD, including systematic Doppler echocardiography and measurement of NT-proBNP and troponin levels.
Given the rarity of HCDD, the optimal treatment approach remains controversial. We have identified abnormal IGH/MAF gene rearrangement results in patients with HCDD, and further studies are needed to determine its role in the development and progression of HCDD. We believe that this test will help us understand the genetic background of the abnormal heavy chain and provide a basis for subsequent treatment. In addition to the renal lesions, our patient exhibited remarkable hematologic findings, which prompted us to administer chemotherapy with satisfactory outcomes. Previous case reports and our own experience indicate that HCDD tends to have a progressive course, with spontaneous remission being unlikely.4, 11, 19 Observational and conventional immunosuppressive therapies appear to have a negative impact on prognosis.20 For patients with multiple myeloma or MGRS, chemotherapy is recommended to improve survival, although its ability to prevent renal progression remains uncertain. Nevertheless, some previous reports have demonstrated a decrease in proteinuria, serum creatinine, and achievement of hematological remission following chemotherapy, suggesting its potential benefit in these patients.21 In our case, standard bortezomib-based therapy was employed, resulting in stable renal function and a significant reduction in proteinuria over a 4-year follow-up period.
To summarize, our case report describes a female patient with nephrotic syndrome (NS) and renal insufficiency associated with γ3-HCDD, who exhibited a positive response to CyBorD therapy and maintained stable renal function over time. However, continuous careful observation is necessary to assess the long-term therapeutic effect. To enhance the renal prognosis of HCDD, a comprehensive understanding of renal pathology and the utilization of advanced diagnostic techniques, such as IGH gene rearrangement assays, are essential for early diagnosis and appropriate treatment. Aggressive chemotherapy may prove beneficial in improving the renal outcome.
AUTHOR CONTRIBUTIONS
Shujun Shi: Conceptualization; visualization; writing – original draft. Kaiying He: Resources; validation; writing – original draft. Shuling Yue: Data curation; formal analysis; supervision. Yaojun Liang: Methodology; visualization; writing – review and editing.
FUNDING INFORMATION
This research was supported by the Natural Science Foundation of Gansu Province (23JRRA0978).
CONFLICT OF INTEREST STATEMENT
None of the authors have financial disclosures relevant to this manuscript.
ETHICS STATEMENT
The study involving a human participant was reviewed and approved by the Ethics Committee of Lanzhou University Second Hospital. The patient provided written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article or supplementary material, and further inquiries can be directed to the corresponding authors.




