Challenges in the Diagnosis of Infantile Enterocolitis and Rare Auto-Inflammatory Syndromes: A Case Report and Literature Review
Funding: The authors received no specific funding for this work.
ABSTRACT
Auto-inflammatory diseases can exhibit a wide variety of clinical symptoms, which often result in delays in diagnosis and treatment for patients. Intestinal inflammation is a clinical symptom frequently observed in auto-inflammatory syndromes. Monogenic auto-inflammatory diseases are a rare cause of chronic intestinal inflammation and early-onset enterocolitis.
1 Introduction
Early-onset enterocolitis is a rare and severe condition that poses significant diagnostic and therapeutic challenges [1]. Immune-mediated inflammatory disorders are an uncommon cause of enterocolitis in early infancy. Auto-inflammatory syndromes are characterized by dysregulation of the innate immune system, resulting in unprovoked episodes of inflammation [2, 3]. The gastrointestinal tract is vital to the immune system, and genetic variations can lead to auto-inflammatory disorders and gastrointestinal symptoms. Chronic inflammation can impact the entire digestive system, from the stomach to the large intestine [4]. Duodenal inflammation typically presents with milder clinical manifestations.
NLRC4 auto-inflammatory syndrome is a rare monogenic disorder. The nucleotide-binding and oligomerization domain-like receptors family (NLR family) is essential in the innate immune response to various pathogenic organisms [1, 2]. The role of NLRC4 was first identified in the immune response to gram-negative bacterial infections, such as Salmonella typhimurium and Shigella flexneri. Mutations in the NLRC4 gene lead to inflammasome activation and dysregulation. This activation triggers caspase-1–mediated processing and secretion of pro-inflammatory cytokines IL-1β and IL-18. A recent study created a mouse model with the NLRC4 V341A mutation, showing increased IL-18 but no intestinal pathologies, suggesting other factors contribute to the disease's gastrointestinal symptoms [5, 6]. Recent studies have also explored the link between NLRC4 inflammasome activation and cancer [2]. The NLRC4 inflammasome may drive colitis-induced inflammation and colorectal cancer CRC through the p53 pathway, which promotes apoptosis in coelomocytes. Studies link the p53 pathway to tumor cell apoptosis, inhibiting tumor progression [7].
Patients with NLRC4 gene variants exhibit a range of clinical manifestations, including cryopyrin-associated periodic syndrome (CAPS) like symptoms, macrophage activation syndrome (MAS) and enterocolitis [3, 4]. This study identifies a missense variant in exon 4 of the NLRC4 gene in a 3-year-old girl with chronic secretory diarrhea and very early-onset enterocolitis. Its main objective is to highlight the significance of monogenic auto-inflammatory syndromes in early-onset enterocolitis.
2 Case Presentation/Examination
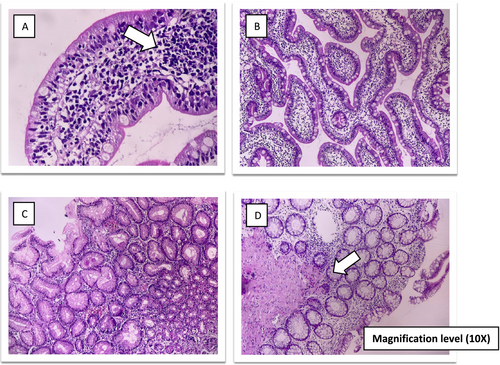
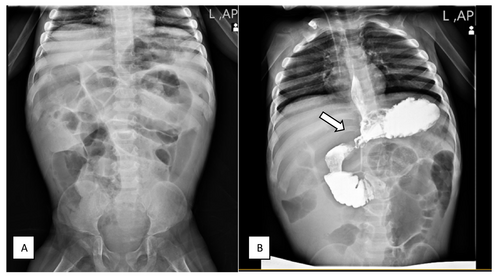
A 3-year-old girl was referred to the gastroenterology clinic for persistent diarrhea that began at 18 months old. She was born preterm at 33 weeks gestation and was hospitalized in the NICU within 9 days after birth. She was born to non-consanguineous parents. Her mother had a history of a miscarriage during the first 3 months of pregnancy. From birth to 12 months of age, she had normal weight gain and growth. She was first admitted to the hospital at 18 months of age for severe diarrhea and growth failure. Diarrhea occurred after the child turned 15 months old. At first admission, she had no fever, vomiting or other symptoms such as skin manifestations and arthritis. The abdominal examination noted abdominal bloating and distension. There was no hepatosplenomegaly. Laboratory findings included metabolic acidosis, hypokalemia and significant thrombocytosis (Table 1). The sweat chloride test results were normal. A colonoscopy and endoscopy were performed. Mucosal biopsy of the esophagus and gastric antrum was normal—sections of duodenal mucosa with preserved villous architecture, revealing non-atrophic duodenitis with increased intraepithelial lymphocytes. Colon mucosal biopsy showed mucosal edema, hemorrhage and a predominant increase of the lamina propria eosinophils (50–60/HPF) with some eosinophilic crypt abscess formation. Colonoscopy findings suggested eosinophilic/allergic colitis. She was discharged with oral prednisolone and shohl's solution. The patient's symptoms improved significantly with oral prednisolone, but diarrhea returned after the dosage was reduced. She had another admission at 23 months of age with fever, vomiting, seizure and exacerbation of diarrhea. She was admitted to the ICU due to severe symptoms, metabolic acidosis and electrolyte imbalances. Elevated acute phase reactants were noted (Table 1). She had a positive test for SARS-CoV-2 infection (COVID-19). Given the multi-organ involvement after COVID-19 infection, the possibility of Multisystem Inflammatory Syndrome in Children (MIS-C) was considered, and the patient was treated with high-dose corticosteroids and intravenous immunoglobulin. At 30 months old, she was hospitalized for abdominal distension, nausea, and vomiting while being evaluated for suspected immunodeficiency. Flow cytometry was used to analyze primary immune deficiencies, and the lymphocyte transformation test was normal. There was no evidence of recurrent infections and abnormality in immunological tests (Table 1). The acylcarnitine profile in plasma by tandem mass spectrometry (MS/MS), high performance liquid chromatography and other metabolic tests were normal. The second duodenal biopsy showed chronic non-atrophic duodenitis with increased intraepithelial lymphocytes (Figure 1). In another admission, she had symptoms of partial intestinal obstruction and ileus. The upper gastrointestinal (UGI) series confirmed the diagnosis of intestinal malrotation (Figure 2). The surgical repair was completed. Following the procedure, she experienced additional admissions for similar symptoms, including diarrhea, intestinal obstruction and severe enterocolitis. Whole exome sequencing (WES) was performed.
| Measure | Reference range | First admission | Second admission | Third admission |
|---|---|---|---|---|
| White blood cell count (WBC; 103μL) | 4.9–12.5 | 7.95 | 13.19 | 15.7 |
| Red blood cells count (RBC; 106μL) | 3.9–5.2 | 5.0 | 4.47 | 5.28 |
| Hemoglobin (g/dl) | 10.9–14.8 | 13.3 | 11.4 | 13.6 |
| Granulocytes (%) | 40–80 | 56.8 | 76.9 | 83 |
| Lymphocytes (%) | 20–40 | 33.3 | 17.9 | 15 |
| Platelet count (PLT; 103μL) | 200–500 | 450 | 924 | 658 |
| Erythrocyte sedimentation rate (ESR; mm/h) | 1–10 | 5 | 38 | 36 |
| C-reactive pProtein (CRP; mg/L) | 0–10 | 1 | 66 | 15 |
| Aspartate transferase (AST; U/L) | < 31 | 34 | 42 | 22 |
| Alanine transaminase (ALT; U/L) | < 31 | 21 | 36 | 25 |
| Ferritin (ng/mL) | 18.1–242.3 | — | 370 | — |
| Immunoglobulin G (g/L) | 453–916 | — | — | 552 |
| Immunoglobulin M (g/L) | 63–275 | — | — | 85 |
| Immunoglobulin A (g/L) | 20–100 | — | — | 70 |
| Anti-Diphtheria toxin Ab (IgG; IU/mL) |
Sufficient protection ≥ 0.1 |
— | — | 0.32 |
| Anti-tetanus toxin Ab IgG (IU/mL) |
Sufficient protection ≥ 0.1 |
— | — | 1 |
| HBs Ab (CLIA) (mlu/mL) |
Protective > 10 |
— | — | 176 |
| Total hemolytic complement (CH50; U/mL) | 51–150 | — | — | 110 |
| Stool pancreatic elastase 1 (μg/g stool) | > 200 | 969 | — | — |
| Urea (mg/dL) | 11–36 | 35 | 51 | 45 |
| Creatinine (mg/dL) | 0.6–1.3 | 0.5 | 1.3 | 1.2 |
| Potassium (meq/L) | 3.5–5.3 | 2.4 | 2.1 | 3.3 |
| Ammonia (μg/dL) | 68–136 | 36 | — | — |
| Sweat Na (meq/L) | — | 15 | — | — |
| Sweat CL (meq/L) | — | 20 | — | — |
| Stool exam (WBC)/HPF | 0–1 | 2–3 | 0–1 | 0–1 |
| Stool exam (RBC)/HPF | 0–1 | 4–6 | 1–2 | 3–4 |
| Calprotectin ag (Fecal; mg/g) | High inflammation > 200 | 422 | — | — |
| Stool fat | Pathologic > 100 | 8–10 | — | — |
3 Methods (Differential Diagnosis, Investigations and Treatment)
Human whole exome enrichment was performed using the twist exome target enrichment kit, and the library was sequenced on the NovaSeq platform with a mean depth of 268X. The sequencing was conducted by CeGaT, Germany. The WES showed a missense variant in exon 4 of NLRC4 (NM-00119139.1, c. 1163G>A, p.Arg388GIn, Chr2:32475770-C-T). The inheritance pattern was autosomal dominant; she was heterozygous for the mutation, whereas her parents were homozygous wild type. A de novo mutation was identified, leading to the consideration of biological therapies, specifically interleukin-1 receptor antagonists.
4 Conclusion and Results (Outcome and Follow-Up)
The patient presented with early onset enterocolitis due to a rare auto-inflammatory disease. Initially, there was no suspicion of this condition, even during suspected intestinal obstruction that led to surgery. Early recognition of auto-inflammatory diseases in cases of enterocolitis can improve prognosis and reduce complications. Recognizing the underlying genetic cause is crucial for guiding treatment decisions and optimizing patient outcomes. However, despite strong pediatrician recommendations for Anakinra treatment, the patient's parents chose not to proceed with it. The patient is receiving short-term, intermittent treatment with prednisolone and taking daily colchicine until the case is reported.
5 Discussion
Most auto-inflammatory disorders are rare, and patients often experience delays in diagnosis and treatment. Intestinal inflammation is a clinical manifestation of auto-inflammatory syndromes. Early-onset enterocolitis may be linked to these syndromes and specific mutations, including Mevalonate Kinase (MVK), nucleotide-binding oligomerization domain 2, and NLRC4 variants [4]. NLRC4 is expressed in the intestinal epithelium, and the NLRC4 inflammasome plays a crucial role in the inflammatory response and the secretion of pro-inflammatory cytokines [2, 3]. There are limited studies on genotype–phenotype correlation and assessment of the clinical features in patients with NLRC4 mutations [8-11]. The mild phenotype of NLRC4-auto-inflammatory syndrome may be similar to familial cold auto-inflammatory syndrome. Patients with milder clinical manifestations experience symptoms such as recurrent urticarial rash, intermittent fevers and Arthritis. A milder variant of NLRC4 inflammasomopathy manifests as cold-induced urticaria, causing hives in response to cold exposure. This form is usually less severe and may not need aggressive treatment.
Symptoms the same as Chronic Infantile Neurologic Cutaneous and Articular Syndrome (NOMID/CINCA syndrome) were reported by a few studies [10, 12]. Severe forms of the disease present with MAS and early-onset enterocolitis [4, 10, 13]. In one study, the initial diagnosis of MAS occurred in a 43-year-old male [14]. MAS, a potentially fatal condition, is a unique clinical manifestation of NLRC4 inflammasomopathy, distinct from other auto-inflammatory disorders. There have even been cases of MAS reported in neonates with NLRC4 mutations [15, 16]. A recent study shows that NLRC4 variants can disrupt immune regulation and lead to auto-inflammation, increasing colorectal cancer risk. The NLRC4 (Ala160Thr) variant may cause recessive immune dysregulation or act as a heterozygous risk factor for ulcerative colitis [3, 7]. These findings highlight the need to include NLRC4 mutations in IBD diagnosis, as they may affect disease characteristics. More research is necessary to comprehend how NLRC4 mutations impact IBD and to create targeted treatments [17].
Table 2 summarizes the literature review. We recently described a patient with very early-onset enterocolitis and a novel NLRC4 gene mutation who did not display MAS or urticarial skin rashes, differing from findings in other studies.
| References | Age at diagnosis | Clinical manifestations | Initial presentation of disease | Laboratory findings | Gene mutations | Treatment |
|---|---|---|---|---|---|---|
| Age at onset | ||||||
| Sex | ||||||
| Romberg, Neil et al. [14] |
1-week-old 1-week-old Male |
Splenomegaly Diffuse alveolar Hemorrhage, enterocolitis MAS |
Fever, secretory diarrhea |
Pancytopenia Hypofibrinoginemia Elevated ESR and CRP levels, hyperferritinemia |
A de novo gain-of-function mutation (p.V341A) in the HD1 domain of NLRC4. | — |
|
43-year-old 1-year-old male |
Fever, acute respiratory distress syndrome, subarachnoid hemorrhage, hematochezia DICa Erythematous rash, Arthralgia (seronegative psoriatic arthritis was diagnosed) |
Recurrent fever Diarrhea, Poor weight gain (gastrointestinal symptoms resolved by one year) |
Pancytopenia hyperferritinemia | A de novo gain-of-function mutation (p.V341A) in the HD1 domain of NLRC4 |
IVIG Corticosteroids, cyclosporine |
|
|
5-year-old 1-week-old male |
Short stature Abdominal pain, Recurrent myalgias |
The severe febrile episode, vomiting, non-hemolytic anemia, acute renal failure | Elevated ESR and CRP levels, hyperferritinemia | A de novo gain-of-function mutation (p.V341A) in the HD1 domain of NLRC4 | — | |
| Canna, Scott W et al. [15] |
7-year-old 6-month-old Female |
Recurrent fever, urticaria-like rash, diarrhea, splenomegaly duodenitis, MASa |
Recurrent fever Poor weight gain |
Elevated ESRb and CRP+ levels, thrombocytopenia Anemia hyperferritinemia |
A de novo missense mutation (c.1009A>T, encoding p.Thr337Ser) affecting the nucleotide-binding domain of the inflammasome component NLRC4 | Colchicine corticosteroids Recombinant IL-1 receptor antagonist (anakinra) |
| Kawasaki, Yuri et al. [16] |
1-month-old At birth Male |
Fever, sever growth restriction, mental retardation, aseptic meningitis, sensorineural deafness, facial appearance typical of NOMID | Febrile immediately after birth, erythema on the oral circumference, palm, foot | — | A novel heterozygous NLRC4 mutation, p.T177A (c.529A>G) | Corticosteroids, recombinant IL-1 receptor antagonist (Anakinra canakinumab) |
| Volker-Touw, N et al. [18] |
— — — |
Recurrent urticarial Skin rash, arthralgia and/or arthritis, irritation of the eyes |
— | — | A novel p.Ser445Pro variant in NLRC4 | Recombinant IL-1 receptor antagonist (Anakinra) |
- a NLRC4: NLR family CARD domain containing 4 protein.
- b ESR: Erythrocyte sedimentation rate, +CRP: C-reactive protein.
Author Contributions
Maryam Khalesi: conceptualization, data curation, investigation. Saeedeh Vahedi: data curation, formal analysis, investigation, resources, software, writing – original draft. Seyedsepehr Jafari: data curation, investigation, validation, visualization. Abdolreza Malek: conceptualization, investigation, validation. Hamid Reza Kianifar: supervision, validation. Mahdieh Vahedi: conceptualization, data curation, investigation, supervision, validation, visualization, writing – original draft, writing – review and editing.
Acknowledgments
We appreciate the assistance of the Clinical Research Development Unit of Akbar Hospital in performing this research.
Ethics Statement
In compliance with the Helsinki Declaration, written and informed assent or consent was obtained from the patient's parent before proceeding.
Consent
All authors reviewed this manuscript and agreed to submit this manuscript. Informed consent for publication was obtained from the patient's parents according to the journal's policy.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.